|
|||||
Soil-Nutrient RelationshipsCation exchangeThe ‘soil cations’ essential for plant growth include ammonium, calcium, magnesium, and potassium. There are three additional ‘soil cations,’ which are not essential plant elements but affect soil pH. The additional ‘soil cations’ include sodium, aluminum and hydrogen. Soil cations that are essential to plant growth
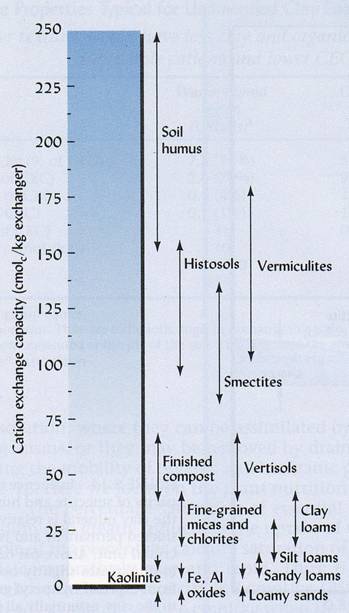
The major distinguishing characteristic of cations is their positive charge. Just like a magnet, a positive charge is strongly attracted to a negative charge. When soil particles have a negative charge, the particles attract and retain cations. These soils are said the have a cation exchange capacity. Although most soils are negatively charged and attract cations, some Hawaii soils are exceptions as we will see. The ‘soil cations’ are further divided into two categories. Ammonium, calcium, magnesium, potassium, and sodium are known as the ‘base cations,’ while aluminum and hydrogen are known ‘acid cations.’ Base Cations
* Unlike the other base cations, sodium is not an essential element for all plants. Soils that contain high levels of sodium can develop salinity and sodicity problems. Acid Cations
The words ‘base’ and ‘acid’ refer to the particular cation’s influence on soil pH. As you might suspect, a soil with a lot of acid cations held by soil particles will have a low pH. In contrast, a highly alkaline soil predominately consists of base cations. Cations in the soil compete with one another for a spot on the cation exchange capacity. However, some cations are attracted and held more strongly than other cations. In decreasing holding strength, the order with which cations are held by the soil particles follows: aluminum, hydrogen, calcium, potassium and nitrate, and sodium.
Anion exchangeIn the tropics, many highly weathered soils can have an anion exchange capacity. This means that the soil will attract and retain anions, rather than cations. In contrast to cations, anions are negatively charged. The anions held and retained by soil particles include phosphate, sulfate, nitrate and chlorine (in order of decreasing strength). In comparison to soils with cation exchange capacity, soils with an anion capacity have net positive charge. Soils that have an anion exchange capacity typically contain weathered kaolin minerals, iron and aluminum oxides, and amorphous materials. Anion exchange capacity is dependent upon the pH of the soil and increases as the pH of the soil decreases. Base SaturationBase saturation is a measurement that indicates the relative amounts of base cations in the soil. By definition, it is the percentage of calcium, magnesium, potassium and sodium cations that make up the total cation exchange capacity. For example, a base saturation of 25 % means that 25 % of the cation exchange capacity is occupied by the base cations. If the soil does not exhibit an anion exchange capacity, the remainder 75 % of the CEC will be occupied by acid cations, such as hydrogen and aluminum. Generally, the base saturation is relatively high in moderately weathered soils that formed from basic igneous rocks, such as the basalts of Hawaii. The pH of soil increases as base saturation increases. In contrast, highly weathered and/or acidic soils tend to have low base saturation. Movement of nutrient from soil to rootThere are three basic methods in which nutrients make contact with the root surface for plant uptake. They are root interception, mass flow, and diffusion.
Nutrient Uptake into the root and plant cellsBefore both water and nutrients are incorporated into plants, both must first be absorbed by plant roots. Uptake of water and nutrients by roots
Once water and nutrients enter the xylem, both can be transported to other parts in the plant where the water and nutrients are needed. The basic outline of how nutrient ions are absorbed by plant cells follows. Absorption of nutrients into plant cells
Nutrient MobilityWithin plantAn important characteristic of some nutrients is the ability to move within the plant tissue. In general, when certain nutrients are deficient in the plant tissue, that nutrient is able translocate from older leaves to younger leaves where that nutrient is needed for growth. Nutrients with this ability are said to be mobile nutrients, and include nitrogen, phosphorus, potassium, magnesium, and molybdenum. In contrast, immobile nutrients do not have the ability to translocate from old to new growth. Immobile nutrients include calcium, sulfur, boron, copper, iron, manganese, and zinc. Within the soilMobility of a nutrient within the soil is closely related to the chemical properties of the soil, such as CEC and AEC, as well as the soil conditions, such as moisture. When there is sufficient moisture in the soil for leaching to occur, the percolating water can carry dissolved nutrients which will be subsequently lost from the soil profile. The nutrients which are easily leached are usually those nutrients that are less strongly held by soil particles. For instance, in a soil with a high CEC and low AEC, nitrate (an anion) will leach much more readily than calcium (a cation). Additionally, in such a soil, potassium (a monovalent cation) will leach more readily than calcium (divalent cation) since calcium is more strongly held to the soil particles than potassium. Silica from minerals also dissolves and leaches from the soil profile during the processes of weathering. It is this dissolution and leaching that transforms primary minerals to the more weathered, secondary minerals that make up the finely-textured soils of Maui. |
|