We certify that we have read this thesis and
that in our opinion it is satisfactory in scope and quality as a thesis for the
degree of Master of Science in Agronomy and Soil Science.
|
|
EVALUATING
MYCORRHIZAL INOCULUM LEVELS IN SOIL
AND QUANTIFYING THEIR
CONTRIBUTION TO THE
PHOSPHORUS NUTRITION
OF COWPEA
A THESIS SUBMITTED TO THE GRADUATE DIVISION
OF THE
UNIVERSITY OF HAWAII IN PARTIAL FULFILLMENT
OF THE REQUIREMENTS FOR THE DEGREE OF
MASTER OF SCIENCE
IN
AGRONOMY AND SOIL
SCIENCE
AUGUST 1980
By
Stephen F. Dowdle
Thesis Committee:
Robert L. Fox, Chairman
Russell S. Yost
Mitiku Habte
Charles L. Murdoch
We certify that we have read this thesis and
that in our opinion it is satisfactory in scope and quality as a thesis for the
degree of Master of Science in Agronomy and Soil Science.
|
|
TABLE OF CONTENTS
LIST
OF TABLES ........................................ 4
LIST OF FIGURES
.......................................
5
INTRODUCTION
..........................................
7
LITERATURE
REVIEW .....................................
9
MATERIALS AND METHODS
................................. 25
RESULTS AND DISCUSSION
................................ 31
APPENDIX
A ............................................
65
LITERATURE CITED
......................................
66
LIST OF TABLES
Table
Page
1 Soil pH, organic carbon, Bray-1 P, NO3--N
and NH4+-N, and
exchangeable
cations in cultivated, noncultivated,
and
subsoil materials ...................................... 26
2 Effect of P fertilizer addition on the P concentration and
P
uptake by cowpea growing in cultivated, noncultivated,
and
subsoil materials ...................................... 39
3 Yield, P concentration, and P uptake by cowpea as affected
by
the addition of phosphate fertilizer in sterile soil
and
estimates of P fertilizer required to compensate for
the
lack of mycorrhizae .................................... 46
4 Nutrient analyses and statistical data for cowpea growing
in three soils with
six levels of phosphorus
fertilization
.............................................. 55
5 Additional nutrient analyses and statistical data
for cowpea growing in three soils with six
levels
of phosphorus fertilization
................................ 65
LIST OF FIGURES
Figure Page
1 Phosphate sorption isotherms for cultivated,
noncultivated, and subsoil
.............................. 28
2 Incidence of mycorrhizal infection in cultivated,
noncultivated,
and subsoil materials as affected by
dilutions
of non-sterile soil material .................. 32
3 Phosphorus and total P uptake by cowpea
growing in cultivated soil material as affected
by soil sterilization and soil P status
................. 36
4 Phosphorus percentages and total P uptake by cowpea
growing in noncultivated soil material as
affected by
soil sterilization and soil P status
.................... 37
5 Phosphorus percentages and total P uptake by cowpea
growing in subsoil matetial as affected by
soil
sterilization and soil P status
......................... 38
6 Yield of cowpea growing in cultivated soil material
as affected by soil sterilization and soil P
status...... 41
7 Yield of cowpea growing in noncultivated soil material
as affected by soil sterilization and soil P
status...... 42
8 Yield of cowpea growing in subsoil material as
affected by soil sterilization, inoculation
with
VA mycorrhizae, and soil P status
....................... 43
9 Percent mycorrhizae infection in roots of plants
growing in cultivated, noncultivated, and
subsoil
material as affected by soil P status
................... 47
10 Potassium percentages in cowpea tops as affected
by
soil sterilization and
soil P status .................... 50
11 Zinc percentages in cowpea tops as affected
by soil
sterilization and
soil P status ......................... 51
12 Sulphur percentages in cowpea tops as
affected by
soil sterilization
and soil P status..................... 52
LIST OF FIGURES (Continued)
Figure Page
13 Calcium percentages in cowpea tops as
affected by
soil sterilization and soil P status
...................... 53
14 Yield of cowpea growing in sterile
cultivated,
noncultivated, and subsoil materials as
affected
by soil P status
.......................................... 58
15 Water retention curves for cultivated,
noncultivated,
and subsoil materials
..................................... 59
INTRODUCTION
Most crop species form
endosymbiotic associations with soil fungi of the family Endogonaceae. Typically, fungal spores germinate, infect
fine roots of host plants, and form characteristic structures, vesicles and arbuscules,
inside the roots. Outside the roots,
mycelia spread profusely in the soil.
The fungus-root structure is known as vesiculararbuscular (VA)
mycorrhizae, or endomycorrhizae.
Although mycorrhizae have
been known to biological scientists for 100 years, agronomists and soil scientists
have only recently focused their attention on mycorrhizae. Several factors were responsible for the
lack of interest heretofore and the current surge of interest. Major obstacles inhibited studies of
practical aspects of mycorrhizae. These
were: (1) difficulties of identification and classification of the fungi and
(2) inability to culture the fungi on synthetic media in the absence of host
plants. The first problem new has a
workable solution while the second appears to be one researchers must live with
at least temporarily. Another hindrance
was one of philosophy; since mycorrhizal fungi were known to be widely
distributed in soil it was assumed that plants were already deriving maximum
benefit from the symbiotic association. Recent research has called this view
into question.
Basic research has
indicated that VA mycorrhizae play a central role in the phosphorus nutrition
of higher plants, particularly when soil phosphorus levels are low, as is often
the situation in soils in the tropics.
Enhanced nutrient uptake is not limited to phosophorus; VA mycorrhizae
also enhance Zn, S, K, and Sr uptake.
In the tropics the human
population continues to grow at a rate which increases pressures on an already
insufficient food supply. New emphasis
is being directed toward increasing food production from tropical soils.
Parallel with needs for accelerated food production is the high cost of fossil
fuels which in turn has increased fertilizer costs. These events have placed a burden on many small farmers who need
fertilizer to increase yields. A search
for ways to increase the economy of phosphate fertilizer has intensified
interest in mycorrhizae.
Agronomists and soil
scientist are challenged to understand the ecology of vesicular-arbuscular (VA)
mycorrhizae to such an extent that they can be utilized to increase crop
yields. Soils in which the mycorrhizal
fungal inoculum level is suboptimal for maximum effectiveness should be
identified and the inoculum level correlated with the benefits bestowed upon the
host by the mycorrhizal association.
The genetic variability within the VA fungi should be quantified and
tested so that inoculation technology may be developed. Such understanding would be helpful in evaluating strain
effectiveness, as well as identifying conditions where insufficient or
inefficient mycorrhizae may be limiting factors for plant growth.
The objectives of this
study are:
(1) To develop a
biological method, to assess the VA mycorrhizae inoculum level and infectivity
potential of soils.
(2) To quantify the
mycorrhizae contribution to the phosphorus nutrition of cowpea growing in three
soils thought to have different levels of mycorrhizae inoculum.
LITERATURE
REVIEW
The term ‘mycorrhiza’
(Gr: fungus root) was coined by Frank to describe associations between certain
non-pathogenic fungi and roots of higher plants. Peyronel et al. (1969) proposed grouping mycorrhizae into three
broad categories: ectomycorrhizae, endomycorrhizae, and ectendomycorrhizae. In ectomycorrhizae, the fungus forms a
compact mantle over the root surface from which the hyphae arise and grow into
the cortex intercellularly.
Endomycorrhizae have external hyphae which are not aggregated to any
great extent and there is extensive growth within the root cortex. Ectendomycorrhizae are similar to
ectomycorrhizae but have both intercellular and intracellular hyphae. Lewis
(1973) suggested grouping ectotrophic and endotrophic mycorrhizae together and
classified them as 'sheathing', 'ericaceous', 'orchidaceous', 'vesicular-arbuscular',
or 'miscellaneous'. Over the past
twenty years it has become evident that the most common and widespread
mycorrhizal infections are the vesicular-arbuscular (VA) type caused by the
Phycomycete group (Nicolson, 1967).
Despite their wide occurrence and ecological importance, only recently
have agronomists and soil scientists begun to recognize the importance of VA
mycorrhizae to the nutrition of crops; such importance is underscored by the
statement of Wilhelm (1966) '... under agricultural field conditions, crops do not, strictly
speaking, have roots, they have mycorrhizae.'
Gerdemann and Trappe
(1975) reviewed the history of the taxonomy of the genus Endogone. The genus was first described by Link in
1809 and later revised by Thaxter (1922). According to this classification, all species form sporocarps and
are distinguished by the structure of the sporocarps and the spores they
contain. Peyronel first suggested in
1923 that Endogone spp. produce VA mycorrhizae, but it was not until the
work of Mosse in 1962 that this was generally accepted (Gerdemann, 1968). The genus Endogone was revised by
Nicolson and Gerdemann (1968) to include species that produce ectocarpic
resting spores. Mosse and Bowen (1968a;
1968b) surveyed 250 samples of Australian and New Zealand soils and some
Rothamsted field soils. They described nine types of spores and devised a key for the identification of Endogone spores using several
diagnostic features: spore attachment, spore contents, spore wall, spore color,
and spore size and shape. Gerdemann and
Trappe (1974) surveyed the Pacific Northwest USA and proposed a new
classification of Endogonaceae that divides the family into five genera: Glomus,
Gigaspora, Acaulospora, Sclerocystis, and Endogone. Endogone is a zygosporic genus and the
only one that does not contain VA endophytes.
The two classification systems just described, Mosse and Bowen (1968a)
and Gerdemann and Trappe (1974), are the most widely used. Hall and Fish (1979) have recently proposed a new key to the
Endogonaceae which was compiled using a computer program. The program assigns weights to the
diagnostic characteristics, assigning high weights to characteristics which
vary least and are easily observed.
As other surveys are
completed, new species are being described. The taxonomy may undergo further
revision to accommodate these additions (Gerdemann, 1976; Redhead, 1977). It should be stressed that the present
taxonomy of Endogonaceae is tentative.
Walker (1979) suggested that researchers place specimens of fungi in
herbaria so that the identification can be checked as taxonomic knowledge
increases. Undoubtably new methods will be developed which will facilitate the
identification of the endophytes.
One promising method is the fluorescent antibody technique which is
being tested on fungi (Malajczuk et al., 1978).
During the past several
years there has been an interest in surveying soils around the world to
determine the presence of VA endophytes.
The resulting information from several continents, encompassing a wide
variety of natural and agricultural ecosystems, has provided insight regarding
the ecological significance of VA mycorrhizae.
Recently two papers have reported the occurrence of VA mycorrhizae in
aquatic plants (Sondergaard and Laegaard, 1977; Bagyaraj et al., 1979a), an
environment where mycorrhizae were previously thought to be absent (Harley,
1969).
When studying the niche
mycorrhizal fungi occupy in a given ecosystem, it is important to understand the adaptations
of the endophytes to that ecosystem. If
more is known about the behavior of mycorrhizal fungi in different environments,
then more sensitive methods can be employed to enumerate the fungi. Three methods have been used to characterize
the inoculum level: 1. extracting and counting spores; 2. direct observation of
infection levels in the plant population; and 3. measuring the rate that test
seedlings become infected (Mosse, 1979).
As Mosse has pointed out, the method selected depends upon the
objectives of the inquiry. Unfortunately
no single method satisfactorily assesses the inoculum level of soils. This deficiency is being filled by modifying
serial dilution techniques and most probable number (MPN) methods in order to
enumerate the viable propagules in the soil (Moorman and Reeves, 1979; Porter,
1979).
Mycocrhizal spores are
usually extracted from soil by wet sieving and decanting (Gerdemann and
Nicolson, 1963). This method leads to
variable results when duplicate samples are handled by two individuals and
besides, the method is laborious when large samples are handled. The method preferentially selects for spores
that are easily extracted (Harley, 969).
Modified procedures involve adding agents to disperse heavy-textured
soils (Sanders, 1976). Differences
between replicate samples analyzed by the wet sieving method may be so great
that statistical comparisons are futile (Nicolson, 1967; Crush, 1973); furthermore, some VA
endophytes produce Spores so small that extraction and counting are
difficult. A flotation-adhesion method
(Sutton and Barron, 1972) resulted in 94-98% efficiency in the recovery of
spores. This method has the advantage of recovering spores regardless of size,
but results in the recovery of organic debris which interferes with spore
counting. Separating spores from organic debris, either by centrifuging in a
sucrose solution (Ohms, 1957) or by differential sedimentation on gelatin
columns (Mosse and Jones, 1968), permits a more quantitative measure of the
spore population. Smith and Skipper
(1979) compared several spore extraction methods and described a new plating
method. Their study points out sources of error in each of the methods studied
and suggests conditions under which one method may be preferred over another. These methods do not distinguish between
viable and nonviable spores and it is this distinction that is necessary for a
practical assessment of the soil inoculum level.
Mosse (1973a) and Tinker
(1975a) have reviewed the work on factors which influence spore populations in
soils. Spore populations are dynamic,
being influenced by season, soil type, soil moisture, light intensity, nutrient
availability and land usage. Whether
these factors influence the fungi directly or indirectly through effects on the
host plant is largely unknown. The
system is complex. Interactions between
the strain of fungi, the host plant, and soil-environment conditions make
generalizations difficult. As one might
expect, the correlation between spore population and infection is strong under
certain conditions and weak under others.
Daft and Nicolson (1972) evaluated three methods for estimating
infection levels in plants and concluded that counting spores produced on
external mycelia was the most accurate and convenient. However, Mosse (1979) has pointed out that
correlations between number of spores and infection are usually good in
experimental situations but much less reliable in situations involving various
soils and various strains of fungi.
Owusu-Bennoah and Mosse (in press) suggested that spore number was
determined by inherent characteristics of the fungi and specific interactions
between the fungi and the soil. In a field inoculation trial involving two
fungal species and three crop plants, they concluded that spore number was not
a good index of infection.
Another difficulty in
relying on spore numbers as a measure of soil infectivity is that spores are
not the only infecting propagules in the soil.
Powell (1976a) showed that hyphae from infected root segments cause infection. Read et al. (1976) surveyed the major
vegetation types in east-central England and concluded that the major source of
inoculum was infected roots or mycelia.
While testing systemic fungicides, Boatman et al. (1978) found that new
roots are infected from mycelia in the soil. Observations such as these point to an additional difficulty:
fungi may have distinctly different life cycles in cultivated and in fallow or
noncultivated soil. Mason (1964) observed
increased spore numbers in a cultivated field as new root growth ceased and old
roots senescenced. Mosse and Bowen
(1968b)
suggested that spores
were formed where root growth is intermittent. In a lowland rainforest in
Nigeria, seedlings were heavily infected while the soil contained no spores at
all (Redhead, 1977). Hayman and Stovold
(1979) surveyed 73 sites in New South Wales and found great variability in
spore numbers. Spore population varied
for the same crop at different sites; they found more spores in agricultural
soils than in native grassland-bush soils.
Thus agricultural field conditions may select sporulating endophytes,
while natural fallow soil conditions may select non-sporulating endophytes.
Soil infectivity can be
semi-quantitatively estimated by examining the extent of infection in sample
plants. Soil infectivity may be defined
as a property of the soil which determines the rate and extent plants form
mycorrhizae. A quantitative measure of
soil infectivity may be possible provided the same host plant is used and careful,
thorough sampling procedures are followed.
The semi-quantitative visual evaluation of infection in the plant roots
needs to be standardized, and even then duplication of results will be
difficult. There are, however,
differences in the ease and the degree in which different host species become
infected. There is also variation in
the amount of vesicles, arbuscules, and hyphae formed by different strains of
fungi. Precisely what to look for when
evaluating infection is a problem.
Hayman (1974) suggested that the total arbuscular formation may be more
important than total infection per se; unfavorable light and temperature
conditions resulting in slow growth of onion was associated with a deficiency
of arbuscules. Nicolson (1960)
developed a root slide technique to quantitatively measure infection. He cut the roots into small segments and
collected the following data: percentage of infection; the number of infected
and noninfected segments; percentage of moribund roots (roots which showed loss
of cortical cells); percentage of external mycelia; the proportion of all
roots which showed mycelia; and the percentage of roots with brown septate
mycelia. Researchers have since used
this technique, often with modifications, to inspect roots for both extent and
intensity of infection. Read et al.
(1976) used the root slide technique to estimate the percent of VA infection by
the expression:
% VA infection= No.
infected segments x 100.
total No. segments examined
They noted that this
procedure describes the distribution of mycelia throughout the root system but
does not describe the intensity of infection in the system. Hayman (1970) attempted to measure both
parameters of infection by recording length of infected root in each segment,
percent root segments with infection, and percent root segments with attached
Endogone hyphae, spores, or vesicles.
Not only are these techniques time consuming but the relationship of the
results to soil inoculum levels is difficult to determine. Strzemska (1974) noted that the occurrence
of VA infection in a given species varied considerably from year to year. If we accept the concept of a dynamic
population of mycorrhizal fungi, then we must concern ourselves with the
implications regarding the infectivity of the soil. The relation of soil inoculum to variation in infection needs to
be investigated.
Giovannetti and Mosse (in
press) compared four methods for evaluating root infection. They compared: 1. the gridline intersect
method; 2. visual estimate of percentage cortex occupied by fungi; 3. estimate
of length of cortex infected from a sample mounted on a slide; and 4. recording
presence or absence of infection on a sample mounted on a slide. They indicated that the visual estimate of
infection, although subjective, can give reliable results. All methods probably overestimate the extent
of infection; because after clearing and staining, roots appear as two
dimensional rather than three dimensional objects.
Measuring the rate a test
seedling becomes infected is rarely reported, although this approach is a
promising method for evaluating soil infectivity. Hayman and Stovold (1979) measured the rate of mycorrhizal
development in clover seedlings in soils from 23 sites. Infectivity of the VA population was not
well correlated with spore population, especially in the native grassland-bush
soils. Moorman and Reeves (1979) made
1/4 and 1/40 dilutions of disturbed and nondisturbed soils. After thirty days corn roots were 77%
infected on the nondisturbed soil but were only 1% infected on the disturbed
soil. The effect of dilution was to
reduce the amount of infection accordingly; however in the disturbed soil this
effect was not apparent until 90 days because of the low inoculum density in
the soil. Porter (1979) adopted a most probable number (MPN)
technique to estimate the infective propagules of VA mycorrhizal fungi. Clover and medic seedlings were planted in
sterile soil which contained serial dilution of sterile and non-sterile soil. The MPN method was designed to be used with
aqueous solutions where the distribution of the organism to be enumerated is
assumed to be spatially uniform and random.
This assumption may not apply in soil where severe clumping of
propagules occurs. The ability of this
technique to generate reproducible results remains to be tested. Nevertheless a
bioassay is needed that detects only the viable propagules in the soil. Such a method would avoid the obvious
difficulties in relying on spore numbers as an estimate of soil inoculum level
or soil infectivity.
The evaluation of
variations in soil inoculum level interests agronomists. Although VA endophytes are present in most
soils, there is evidence that the level is suboptimal under certain conditions. Further research is needed to identify these
conditions. Ross (1979) has observed
that colonization of soybean roots by naturally-occurring mycorrhizal fungi is
lower compared with inoculated soybeans which are grown in sterile soil. He concluded that low sporulation of these
fungi in field soil probably results in low inoculum level for subsequent
crops. The inoculum level in some
Nigerian soils was so low that Stylosanthes guyanensis seedlings
did not become infected during the course of the experiment (Mosse, 1977). However S. guyanensis does not
appear to be very mycotrophic, thus it is probably a poor indicator of soil
inoculum levels.
The standing vegetation
or the preceding crop may have an impact on the soil inoculum level. Khan (1972) made use of this fact and
transplanted infected and noninfected maize seedlings into unfertilized plots
which had previously been occupied by weeds of the Chenopodiaceae family,
reported to be non-mycorrhizal (Gerdemann, 1968). P uptake and dry weight of mycorrhizal plants were much greater
than the controls; grain weight was almost 12 times greater on mycorrhizal
plants. In a study designed to measure
the rate of spread of an introduced VA fungi, the effect of growing
nonmycorrhizal plants in the soil was to reduce the vigor of the indigenous
fungi thereby enhancing the spread of the introduced species (Powell,
1979b). Kruckelmann (1975) found that
fertilizers, soil tillage, and crop rotations affected the number of spores in
arable soil. His results showed spores were more frequent in loamy soils than
in sandy soils. Spore populaticn
correlated better with pH than with K, carbon,
or nitrogen content of
the soils. Spore numbers increased with
higher pH values and decreased with increasing phosphate contents. It certainly would be desirable to know what
effect, if any, flooding the soil has on the soil infectivity.
In citrus culture, and
some other perennial plantation crops, it is a common practice to fumigate soil
or use sterilized growth media to grow seedlings. Heavy P fertilization is necessary to relieve stress resulting
from the lack of mycorrhizae (Kleinschmidt and Gerdemann, 1972). Under these conditions inoculation with
mycorrhizal fungi can partially substitute for P fertilization (Menge et al.,
1978).
The importance of
mycorrhizae for eroded lands has not been experimentally determined. However there are several reports on the
vertical distribution of mycorrhizal spores in the soil. Sutton and Barron (1972) found that the
number of spores changed little with soil depth to 16-24 cm, but declined with
further increase in depth. Spores
occurred mostly in the top 15 cm of soil in Nigeria (Redhead, 1977). The mean number of spores per 500 cm3 at various depths were
2 cm, 748; 7.5 cm, 1946; 15 cm, 1064; 30 cm, 55. Spore numbers in eroded soils were 25% of adjacent non-eroded
sites, and a response to inoculation was obtained in 8 out of 10 eroded soils
(Hall and Armstrong, 1979). In soils
disturbed by strip mining operations the inoculum level was suboptimal (Reeves
et al.,1979). Daft et al.(1975)
postulated that a mycorrhizal association may be essential for the survival of
most herbaceous plants growing in coal spoils.
They obtained a significant response to inoculation. The implication is that where the surface
soil has been removed the inoculum level of exposed soil may be suboptimal for
plant growth.
Effect
of VA Mycorrhizae on the Host Plant
Increased phosphate
absorption by plants infected with VA mycorrhizae when compared with
noninfected plants and the increase in P concentration in plant tissue has been
well established (Mosse, 1973a; Tinker, 1975a). Although reports of increased uptake of other nutrients and
increased water absorption possibly indicate multiple effects of mycorrhizae on
plant nutrition, nearly all host growth responses have been attributed to
improved phosphorous nutrition.
Experiments using
insoluble phosphates have demonstrated that enhanced growth and P uptake was
associated with mycorrhizal plants (Murdoch et al., 1967). It has been inferred by same investigators
that mycorrhizal fungi may possess P-solubilizing mechanism by which
mycorrhizal plants utilize forms of P unavailable to nonmycorrhizal
plants. Although no such mechanisms
have been demonstrated there is data to suggest that mycorrhizal plants absorb
sparingly soluble P more readily than nonmycorrhizal plants. Working with a high P sorbing soil in
Hawaii, Yost and Fox (1979) indicated that the threshold concentration for P
uptake (the concentration of P in the soil solution below which no P is
absorbed) is lower for mycorrhizal plants.
Data from Cress et al.(1979) raises the possibility that a major factor
contributing to the increased uptake of phosphorus by mycorrhizal plants is a
greater ion affinity by the mycorrhizal absorbing sites. The relative importance of this increased
ion affinity in soil situations where diffusion of phosphorus is rate limiting
can not be determined from their study. Jackson et al.(1972) studied
utilization of rock phosphate and did not observe a response to inoculation
with VA fungi unless the rock phosphate were mixed in the soil, indicating the importance of
the spatial proximity of the association and the nutrient source.
To identify the source of
P for mycorrhizal and nonmycorrhizal plants, soil was labeled with 32P
and the specific activity of absorbed P in infected and noninfected
plants was determined. The results
indicated that mycorrhizal and nonmycorrhizal plants obtain phosphorus from the
same source (Sanders and Tinker, 1971).
Such data support the idea that the effect of mycorrhizae results from
the hyphae forming a better distributed surface for absorbing phosphorus than
roots alone.
Hattingh et al.(1973)
provided direct evidence of hyphal uptake and translocation of phosphorus. 32P-labeled phosphate which had
been placed 27 mm from the root surface was absorbed when the roots were
mycorrhizal; when the hyphae were severed mycorrhizal roots did not differ
significantly in content of 32P-labeled phosphate content from
nonmycorrhizal roots. Growth chamber
results such as these should be interpreted with caution. It is probable that hyphal growth is more
profuse because of conditions on the soil plane (Hattingh, 1975). Owusu-Bennoah and Wild (1979) used
autoradiography to demonstrate phosphate depletion zones around mycorrhizal and
nonmycorrhizal roots. They concluded
that the main increase of phosphate uptake by mycorrhizae was from soil within 2 mm of the root
surface. However the experimental
conditions of such work demand a closer look before extrapolations are made to
other soil-plant systems. Finely
crushed soil with small pore spaces which may be water saturated probably
restricts hyphal growth. The increased
absorbing; surface of fungal hyphae is important as well as the distribution of
absorbing surface in the soil. The
specific interaction between the fungal strain and the soil properties will
affect the relative importance of these two parameters.
The diffusion of
phosphorus in soil and uptake by plants has been studied in detail. Bhat and Nye (1974) indicated that a
phosphorus depletion zone surrounds the active absorbing root; hence the value
of external mycelia may be that they extend beyond the depletion zone and
absorb phosphorus in non-depleted soil.
Realization of this prompted Rhodes (1979) to write "...nutrients
most likely to be involved in plant growth responses to VA mycorrhizal
infection are those for which the rate-limiting step for uptake by plants is
movement to roots through soil by diffusion."
Properties of mycorrhizal
hyphae are becoming better understood. Pearson and Tinker (1975) demonstrated P
transport and measured a mean steady state flux of P in the external hyphae of
0.3-1.0 x 10-9 moles cm-2s-1. They
did not determine a value for absorbing power of the hyphae per unit
length. Cooper and Tinker (1978)
studied the uptake and translocation of P, Zn, and S. In clover external hyphae translocated molar amounts of P, Zn,
and S in the ratio of 35:5:1 and the mean fluxes in the ratio of 50:8:1 which
suggests
high relative efficiency in the uptake and translocation mechanisms for
P. Their results also indicated that
the phosphorus demand of the host affected the flow of P
in the hyphae, and that
the amount of external hyphae in the soil (i.e. the total hyphae length) was
secondary in importance.
From an ecological
perspective, Baylis (1975) suggested that mycorrhizal fungi have exercised a
controlling influence on the evolution of roots. He submitted that magnolioid roots are more dependent on
mycorrhizae for P uptake in low P soils than are graminoid roots. Magnolioid roots are coarsely branched and
the ultimate roots are rarely less than 0.5 mm in diameter. The roots have a compact stele and normally
do not have root hairs. Graminoid roots
are finely divided, with ultimate branches often less than 0.1 mm in diameter.
They are densely covered with root hairs 1-2 mm in length. Data from Yost and Fox (1979) lend support
to this hypothesis. Tinker (1975b) went
a step further by pointing out that root hairs may be less effective than
hyphae because root hairs have short lives and inter-hair competition is keen;
hyphae are more dispersed and hence will have fewer overlapping P depletion
volumes.
Evaluating the
mycorrhizae-legume symbiosis is particularly challenging. Legumes may play a central role in
increasing food production in tropical soils.
Because of their ability to obtain nitrogen through symbiotic
association with Rhizobia, it may be possible for farmers to obtain good
yields with a minimum of expensive chemical fertilizers. Symbiotic nitrogen fixation by legumes may
have a high P requirement (Munns, 1977).
Phosphorus content of nodules may be 2-3 times more than the P content
of the roots on which they are formed (Mosse et al., 1976). Also other micronutrients, notably Cu and Zn,
have been shown to enhance or be necessary for nodulation (Hallsworth, 1958;
McIlveen et al., 1975). It is not
surprising that the, literature reports instances of legumes halving better
nodulation, higher nitrogen percentage, and greater nitrogenase activity when
they were inoculated with VA mircorrhizal fungi (Abbott and Robson, 1977;
Mosse, 1977; Daft and El-Giahmi, 1976).
Bagyaraj et al. (1979b) attempted to directly test the effect of VA
fungi on N-fixation and plant growth.
Four inoculation treatments were used: 1. uninoculated control, 2.
inoculated with Rhizobium japonicum, 3. inoculated with a VA
fungus Glomus fasciculatus, and 4. inoculated with Rhizobium
and Glomus. After 60 days nodule
mass and nodule nitrogen content from treatment 4 were double that from
treatment 2. Shoot dry weight from
treatment 4 was increased 64% over treatment 2; however the increase in grain yield
was not significant (at P = 0.05). Waidyanatha et al. (1979) found that
inoculation with VA fungi stimulated nodule weights and nitrogenase activity
far more than plant growth. They
believed that this effect on N fixation may be the most important effect of
mycorrhizae on legumes.
The relative progression
of Rhizobium nodulation and mycorrhizal infection is not known. The first infection units in soybean
seedlings appeared 10-12 days after planting which corresponded with the
appearance of root nodules (Carling et.al., 1979a). Cox and Sanders (1974) defined an infection
unit to include the internal mycelia relating to a single entry point. In another study Carling et al. (1979b)
worked with nodulating and non-nodulating isolines of soybeans. Total plant and nodule dry weight and
nitrate reductaset and nitrogenase activities were increased significantly in
mycorrhizal, nodulating plants as compared to nonmycorzhizal nodulating
plants. When phosphorus was substituted
for mycorrhizae, similar growth and enzyme activities were observed. They concluded that the effects resulted
from an improved nutritional environment for the plant rather than a direct
interaction between the fungus and the bacterium. These results emphasize that our knowledge about the
mycorrhizosphere is limited. The
competition and the synergism among mycorrhizae, Rhizobia, and the host
plant apparently are complex and are not adequately understood at present.
The significance of
mycorrhizae in plant nutrition is probably greatest when fertility is low. Generalizations about the role of
mycorrhizae in high fertility situations are more difficult to make. There
appears to be a critical value of soil phosphorus for each species in certain
growth conditions above which plants will grow well without mycorrhizae. This critical value may be determined in
part by the diffusion rate of phosphorus in a particular soil (Cooper, 1975).
However it appears that it is the concentration of P in the plant that
regulates the mycorrhizal association rather than the concentration of P in the
soil. Sanders (1975) induced high P concentrations
in the plant by foliar feeding which inhibited infection. Menge et al. (1978a) used a 'split root'
technique to demonstrate that number of spores, vesicles, arbuscules, and
hyphae were not influenced by high soil P levels but were negatively influenced
by high concentrations of P in the root.
The physiology of the
root is likely to change as the percentage phosphorus content increases. This may be a self-regulatory mechanism of
the plant. Ratnayake et al. (1978) examined
root exudates, phospholipid content of root tissue, root P content, and
membrane permeability. They concluded
that a major consequence of low P nutrition is a decline in membrane
phospholipids, an increase in membrane permeability, and increased exudation of
metabolites. With increased P nutrition
membrane permeability decreases as does the exudation of metabolites.
Growth depressions
resulting from inoculation with VA
mycorrhizae have been reported. Mosse
(1973b) attributed this to phosphorus toxicity and specific interactions
between the soil and the endophyte.
However there are reports of growth suppresions when P toxicity was
clearly not a problem. Cooper (1975)
suggests the suppresions were related to the P status of the soil: at high
phosphorus levels little infection develops and there is no growth response to
mycorrhizae; at low levels of soil P a large response to fungi occurs because
of an overriding improvement in the P nutrition of the plant; at intermediate
levels, fungal infection is infrequent and transient reductions in growth occur which are followed by increases in
growth response to the fungi with time as infection increases and P demand
increases. A similar line of reasoning
was pursued by Yost and Fox (1979) who suggested that at intermediate soil P levels
decreased P uptake can occur if, with increasing levels of soil P,
effectiveness of the endophyte decreases faster than P uptake increases by the
uninfected root. Sparling and Tinker (1978) reported a decrease in shoot weight
of three grasses which had been inoculated with VA mycorrhizae; they thought
the decrease was understandable in light of the branched root system with many
root hairs and relatively small P requirement.
Although there are many
aspects of VA mycorrhizae that are not understood, research is moving ahead
rapidly-toward developing inoculation techniques, testing relative efficiency
of fungal strains, evaluating field inoculation trials, and attempting to
define soil and plant conditions when a response to inoculation may be
expected. The objective of this study
was to characterize the soil inoculum level of some soils and to relate this to
the quantity of phosphorus contributed by mycorrhizae to plants.
MATERIALS
AND METHODS
Cowpea (Vigna unguiculata
L. TVu 3563) was grown in 10 cm pots (525 g oven dry soil) in a glass house. The Wahiawa soil used (a Tropeptic
Eutrustox) was collected from three sites on the Poamoho Experiment
Station. The Wahiawa soil is deficient
in available phosphorus for most crop species.
I was presumed to have different levels of native mycorrhizal
fungi. Cultivated surface soil, 0-10 cm
depth, was collect from a field which has been cultivated intermittently for
at least ten years. Subsoil was
collected at 120 cm dept from the same site. Surface soil, 0-10 cm depth, was
collected from a site that has not been cultivated for at leas fifteen
years. This site had been occupied by
various perennial grasses.
The soils were
characterized by the following measurements: pH (l:l soil to distilled water
ratio), organic carbon (Walkley-Black method, 1935), Bray-1 P, NO3-
and NH4+ (1N KCl soil extraction with distillation on
Micro-Kjeldahl apparatus), and exchangeable cations (1N NH4OAc soil
extraction with Ca, K, and Na determined by
flame emission spectrophotometry and Mg determined by atomic absorption spectrophotometry. The results are presented in Table 1.
Experiment
1: Soil Inoculum Bioassay
All soil materials were
passed through a 3/16 in. screen.
Phosphorus was added to each soil in amounts that were determined by P
sorption curves (Fox and Kamprath, 1970).
The P sorption curves are presented in Fig. 1. Phosphorus was added as KH2PO4 to each soil
to bring the level of P in the soil solution to .025 mg/liter. This level of soil P was chosen so that most
soils could be brought to a standard P level
|
|
and thus the test plant
growth and infection would more closely reflect the inoculum level. Soil pH was adjusted with CaC03
to about 6.5. Zinc (10 kgZn/ha as ZnS04·7H20)
was added to each soil.
A portion of each soil
was sterilized by Y-irradiation (1.5 Mrad) from a 60Co source. This exposure is approximately twice the
dose of irradiation reported by other researchers, and was used to ensure
complete sterilization (Pearson and Tinker, 1975). The non-sterile soil was 'diluted' by mixing with various amounts
of sterile soil. The proportions of
non-sterile to sterile soil were 1/0, 1/3, 1/9, 1/27, 1/81, 1/243, and
1/729. There were four pots for each
treatment. The dilutions were made by
weighing the appropriate amount of sterile and non-sterile soil and mixing the
soil in a mechanical soil mixer for 5 minutes.
The highest dilutions were mixed first to avoid contamination from less
diluted soil materials. After mixing
each treatment the soil mixture was divided into four pots.
Three seeds were planted
per pot which were later thinned to 1 plant per pot. The plants were harvested 22 (Phillips and Hayman, 1970) and examined in
an open petri dish with a dissecting microscope for the presence or absence of
mycorrhizal infection. Stained
preparations for roots of plants grown in cultivated and subsoil materials were
derived from the entire root system.
However, due to a greater root mass, only half of the root system of
plants in the noncultivated soil was adequate for this purpose. The samples were evaluated by enumerating
the incidence of mycorrhizal infection. An incidence of infection was defined
as a continuous area along the root where vesicles, hyphae, or arbuscules were
observed.
|
|
Experiment
2: Quantifying the P Contribution by Mycorrhizae
Cowpea (Vigna unguiculata
L. TVu 3563) was grown in 2½ gallon plastic pots (9 kg OD soil) in the
glasshouse. The three soils used in
this experiment were collected from the same sites as in experiment 1.
Soils were passed through
a ¼ in. screen. Rates of P added to
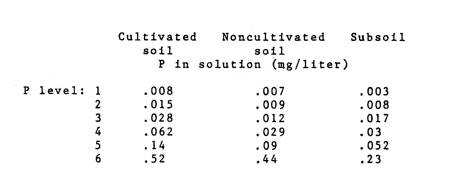
each soil were determined from the P sorption curves presented in Fig. 1. The following levels of P in the soil
solution were established for each soil:
Phosphorus was added as
KH2P04. Potassium
(as KCl) was added to each soil in varying amounts to equalize the amount of K
in each pot. Soil pH was adjusted with
CaC03 to about 6.5. Zinc (10
kgZn/ha as ZnS04·7H20) was added to each soil. Nitrogen (as NH4NO3) was added to each soil to
bring extractable NH4+ and NO3--N
levels to 35 mg/liter for all soil materials in order to provide for equal N
availability among the three soils.
Six levels of P were
established in each lot of soil that remained non-sterile, and in each lot of
sterilized soil (Y-irradiated with 1.5 Mrad).
In addition to the nonsterile and sterile treatments there was one
mycorrhizal inoculation treatment in the subsoil. In the pots containing non-sterile subsoil, one-gram samples of
fresh mycorrhizal cowpea roots were placed approximately 1 inch below the
seed. The inoculum was obtained by
growing cowpea in soil material collected from the small plots at the Mauka
campus research facility. There were three replicates for each treatment,
giving a total of 126 pots.
Pots were arranged on benches
in the glasshouse in a completely randomized design. Seven seeds were planted per pot; these were later thinned to
three plants per pot. Seeds were
inoculated with Rhizobium strain Nit: 176 A22 of the Cowpea group. Pots were placed in plastic basins
containing water and the plants were watered by capillary rise. This method of
watering was chosen to avoid contaminating the sterile soil by splashing water
from one pot to another.
Plants were harvested 38
days after planting. Whole tops were
oven dried, weighed, and the nutrient composition was determined by x-ray
emission spectroscopy. Mycorrhizal
infection was evaluated on samples of fine roots. The soil was washed from the entire root
system of the plants in each pot. Four
samples, approximately 8 cm long and 2 cm wide, were cut from the root system;
two samples were cut from either side of the root system 4 cm below the root
crown, one sample was cut from the center of the root system 10 cm below the
root crown, and one sample 16 cm below the root crown. The root samples were cleared and stained as
in experiment 1, and examined in an open petri dish with a dissecting
microscope. Mycorrhizal infection was
semi-quantitatively rated on a scale of 0-100%.
RESULTS
AND DISCUSSION
I.
Soil Inoculum Bioassay
A serious limitation to
research with VA mycorrhizae is the lack of methods to determine the
infectivity potential of soils. Using
the soil dilution method described, differences were detected among the
mycorrhizae inoculum levels of various soil materials (Fig. 2). Incidence of infection was greatest in the
undiluted (1/0), noncultivated soil and least in the subsoil. As the non-sterile soil was diluted with
sterile soil, the incidence of infection declined to a level that was not
affected by further additions of sterile soil material. In general, for all soil materials, an
absolute extinction point, a dilution with sterile soil until no infection
occurred, was not observed. The proportion
of non-sterile soil at which there was no further decrease in infectiveness was
1/3, 1/9,and 1/27 for the subsoil, cultivated, and noncultivated soil material
respectively. The reciprocals of these
proportions give the following indexes of infectivity for the respective soil
material: 3, 9, and 27. The significance
of such 'base levels' is not clear.
This observation should be verified and then studied in greater detail.
The concept of 'incidence
of infection' is introduced here to differentiate from 'infection unit' which
was described by Cox and Sanders (1974) as the internal mycelia relating to a
single entry point. Using this bioassay
procedure
the identification of the source of infection (i.e. the infecting propagule or
propagules) was not critical to detecting differences in soil inoculum levels; contrary
to the enumeration of infection units where points of entry must be determined
to differentiate each infection unit.
What was observed in the roots of the test plants was the initial
penetration and early development within the roots of hyphae; extensive
development of infection within the root had not
|
|
occurred. Incidences of infection were observed in
discrete areas in the root; most often infections were observed as either a
piece of penetrating hyphae, arbuscules, or vesicles. Enumeration of incidence of infection in diluted soil materials
where infection was less developed was more accurate than in undiluted soil
materials where the spread of infection was more extensive.
In a bioassay of this
type it is important that the growth of the indicator plants be as uniform as
possible so that results are comparable. It was apparent at the end of the
growing period that plant growth in the three soils was not equal. Root development, and to a lesser extent
shoot development, were greatest in the noncultivated soil material. As a result, a greater volume of soil was
explored in the noncultivated soil. To
some extent this difference was mitigated by expressing incidence of infection
on a per gram root weight basis.
The timing of harvest was
critical. Soil systems are dynamic;
roots are growing, spreading through the soil, encountering viable propagules
and becoming infected. Not only do the
number of viable propagules determine the final extent of infection, but also
the abundance of susceptible roots. For
this reason it is important to have an equal production of roots in all soils
being tested. Methods should be
developed that minimize differences in root growth. Stanford and DeMent (1957) devised a method for measuring
nutrient absorption using predeveloped standard root mats. Seeds were planted in sand cultures in
bottomless cardboard cartons which were nested in a second carton with the
bottom intact. At the end of the
initial growing period a mat of roots had formed at the bottom of the carton. The bottomless cartons were then removed and
the roots were placed on the soil materials for fertility evaluation. The relevance of this method to the
inoculum bioassay is that differences in root biomass were minimized. Unfortunately in this bioassay there were
unexpected differences in the growth of the host plant in the three soil
materials. Differences began to appear
ten days after planting. Seedlings in
the noncultivated soil were taller, and first true leaves began to appear earlier
than in the other soil materials.
Growth of seedlings in the cultivated and subsoils was similar. Reasons for these differences will be
discussed later, but one thing is apparent the chemical and physical properties
of test soils should be normalized to the degree possible.
The first adaptation of
the principles of serial dilution to soils was reported by Tsao (1960) who
attempted to estimate the infectivity of soil with respect to Phytophthora
fungi. His rational of the method was
that serial dilutions of infected soil, with sterile soil as the dilutent,
would eventually reduce the disease potential to zero. One aspect of the method used in this thesis
which could be improved is the method of dilution. Because of the relatively large volume of soil used (525 g 0D
soil/pot) it was believed that mixing and preparation must start with the most
dilute mixture to avoid contamination.
For this reason serial dilutions of soil were not made. An interesting approach to soil dilution was
recently reported by Porter (1979). Using a smaller volume of soil he serially
diluted non-sterile soil with sterile soil and then placed the mixtures into
the center of pots containing sterile soil.
Two seeds of clover were planted over each soil mixture and after six
weeks the roots were examined for the presence or absence of mycorrhizae. The number of infective propagules was
estimated using standard microbiological most probable number (MPN) techniques
(Cochrane, 1950). The estimates using
MPN tables were greater than estimates using the wet sieve method, particularly
for endophytes with hyphal diameters less than 3 um. The comparisons of these methods were made using two soils, a
sandy clay and a sandy loam. This is
significant because the wet sieving method is well adapted for use on coarse
textured soils where spores are more readily extracted. Also, serial dilutions, as used in soil
investigations, have a greater chance of success where clods and strong
aggregation are not factors. It needs
to be determined whether the dilution method can give reliable results in high
clay soils where spore extraction is difficult and clumping may interfere with
the assumption about the random distribution of the test organism.
II.
Quantifying the P Contribution of Mycorrhizae
Effect
of VA Mycorrhizae and Soil P Level
The response of cowpea to
soil phosphorus levels was strongly influenced by whether or not plants grew in
sterile or non-sterile soil. The P
concentration of whole tops and P uptake by plants growing in cultivated,
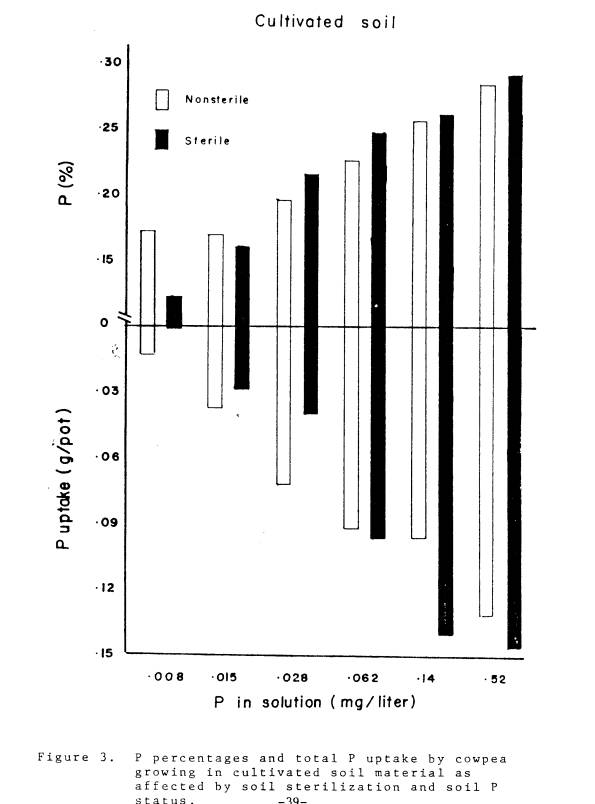
noncultivated, and subsoil are shown in Fig. 3-5. As soil P levels increased, the concentration of P in
plant tissue and total P uptake increased (Table 2). The relative advantage of native mycorrhizae versus no
mycorrhizae was greatest in the noncultivated soil, and least in the subsoil.
This was manifested at the lowest soil P levels where total P uptake in
nonmycorrhizal plants was 1, 9, and 56% of the naturally infected plants in
the noncultivated, cultivated, and subsoil respectively. Total P uptake in the nonmycorrhizal plants
in the subsoil was only 22% as much as the inoculated plants. It is tempting to
explain these results in terms of differences in soil inoculum density; however
inoculum density was only one of the contributing factors, albeit an important
one.
Growth, P concentration,
and P uptake by nonmycorrhizal plants were greater when compared with
mycorrhizal plants at higher soil P levels with two notable exceptions: 1. the
P concentration in mycorrhizal plants in the noncultivated soil material
remained higher than in the nonmycorrhizal plants at all levels of soil P; and
2. the P concentrations of inoculated plants growing in the subsoil material
were higher than plants growing in sterile or non-sterile subsoil material at all
levels of soil P.
|
|
|
|
|
|
Ultimately agronomists
and soil scientists are interested in the yield response as affected by
mycorrhizae (Fig. 6-8). The response
curves resemble the hypothetical growth response curves for mycorrhizal and
nonmycorrhizal plants discussed by Mosse (1979). The relative advantage of mycorrhizal plants over nonmycorrhizal
plants disappeared at approximately .062 mg P/liter, .029 mg P/liter, and .008
mg P/liter for the cultivated, noncultivated, and subsoil materials respectively. In the subsoil, the differences in yield
among all plants, those inoculated and those in sterile and non-sterile soil,
are small. The trends, if any, are not
distinct. When soils are irradiated, as
in this experiment, not only are the mycorrhizal fungi eliminated from the
soil, but so are all microorganisms.
The intersection of the response curves for plants grown in non-sterile
and sterile soil probably also reflects the degree to which pathogenic
organisms are inhibiting growth. If
there were a high population of nematodes, for example, yield in non-sterile
soil may be depressed and the curves may intersect at a lower level of soil
P. In this experiment, pathogens did
not appear to be a factor in nonsterile soil materials. The soil P level where the growth response
curves intersect, termed the critical P level for mycotrophy (Cooper, 1975),
reflects several soil characteristics, inoculum density being only one of them.
The significance of differences in inoculum densities are not
easily determined. Are the differences
between the noncultivated and cultivated soils, for example, significant to the
growth of a crop? The answer to that
question may depend upon the soil as well as the inoculum. Daft and Nicolson (1969) conducted an inoculum
density experiment in pots and found that even low levels of inoculum, 3 spores
per plant, were able to effect complete colonization of roots. No significant difference in growth response
occurred among the various inoculated treatments. However because of the confining conditions in pots, the
relevance of these findings to
|
|
|
|
|
|
field conditions cannot
be assumed. It is quite probable that
in field situations a higher inoculum density is needed for maximum growth
response. Working with pot cultures, Carling et al. (1979a) found that the
number of infection units in 21 day-old soybean seedlings was dependent upon
inoculum density. This suggests that
inoculum density of mycorrhizae-forming fungi may be particularly important in
early seedling establishment. This may
account for the improved establishment of clover in pastures after inoculation
with VA fungi (Powell, 1977; 1979). The
slow development of mycorrhizae in seedlings may account for the observation
that the nutritional requirement of seedlings for P is much greater than after
the plants are established. The forage
legume Desmodium aparines required about 0.2 ppm P in solution
for establishment, but .01 ppm P was adequate for regrowth after harvest (Fox
et al., 1974). It is evident that
conditions under which differences in inoculum density will be significant to
the ultimate growth response of a crop need to be defined. It is not known whether high infection
levels in the seedling stage are a requisite for maximum growth responses.
The amount of phosphorus
required to bring the level of soil solution P to the critical level of
mycotrophy was different for the three soil materials. A farmer who must add phosphate fertilizer
to the soil in order to sustain yields might well ask of what value are the
mycorrhizae? In Table 3, yield, P
concentration, and P uptake are presented relative to the amount of P
fertilizer added to the soil. The
amount of phosphate fertilizer needed to compensate for the lack of mycorrhizae
can be estimated from this data. For
example, in non-sterile cultivated soil with 0 P added (.008 mg P/liter), yield
was 7.7 g/pot. When mycorrhizae were
eliminated from the soil, similiar yields were theoretically possible (Fig. 6)
if the soil solution P was increased to approximately .01 mg P/liter. From the P sorption curve, the soil requires
an addition
of approximately 40 kg P/ha to raise the level of P in the soil solution to .01
mg P/liter. Similar calculations were made for all three
soils using P% and P uptake as the indicators of the mycorrhizal effect and
the estimates are presented in Table 3.
The range of estimates were 40-140 kg P/ha, 220-440 kg P/ha, and 10-100
kg P/ha for the cultivated, noncultivated, and subsoil materials
respectively. When the inoculum density
was increased by inoculation with mycorrhizal fungi, the estimate of the amount
of P necessary to compensate for the lack of mycorrhizae increased (Table 3).
This method can also be
used to estimate the amount of fertilizer P for which mycorrizae can substitute
(Menge, 1978). Methods such as these,
though imperfect, represent an attempt to quantify the mycorrhizal benefit to
the host. In addition such methods may
contribute in the evaluation of different strains of mycorrhizal-forming fungi,
particularly strains that are morphologically similar. At present, there are no sure methods that
can measure the effects of native mycorrhizae to the host plant.
The differences in
inoculum level in the three soils were reflected in the extent of colonization
of the roots by mycorrhizal fungi (Fig. 9).
Because of the semi-quantitative nature of the evaluation of infection,
the trends in Fig. 9 are more important than the actual percentages. In plants growing in the subsoil, the native
mycorrhizal fungi did not colonize the roots to the same extent as roots were
colonized in the cultivated and noncultivated soils. Infection was also somewhat less in
the cultivated than in the noncultivated soil materials. With increasing levels of soil P the extent
of infection declined, with the exception of plants inoculated with VA
mycorrhizae. The extent of infection in
the inoculated plants remained high in spite of increasing levels of soil and
plant P. The fact that the plants
continued to increase P uptake even though the yield response to P had ceased
indicates that the mycorrhizae were still active. It is interesting to observe that mycorrhizae infection in
naturally infected plants did not cease altogether at high soil P levels. What effects, if any, mycorrhizae have on
plants with adequate P nutrition warrants further study.
|
|
|
|
Previous work exploring
the mechanisms which regulate the mycorrhizae have established that mycorrhizal
infection is attuned to the phosphorus nutrition in the plant, rather than the
phosphorus level in the soil (Sanders and Tinker, 1975; Menge et al.
1978). The hypothesis that phosphorus
inhibition of mycorrhizae is associated with a decrease in root exudation and
associated changes in root membrane permeability is particularly interesting in
light of this study (Ratnayke et al., 1978). During early autotrophic growth,
seedlings are dependent upon seed reserves for nutrition and relatively independent
of the nutrient status of the soil.
During this stage, seedlings may have the same susceptibility to
infection, regardless of soil nutrient levels.
As the phosphorus concentration in the plant increases,
presumably the control mechanism in the plant exerts some unspecified
influence which inhibits development of mycorrhizae. It may be that the mycorrhizae-forming fungi in the inoculum was
a different strain than the native fungi in the soil materials used in this experiment,
and that this strain responded differently than the native fungi. The native fungi was inhibited by high P concentrations
in the plant, whereas the mycorrhizae in the inoculated plants maintained a
heavy colonization despite high P concentrations in the plant. Mycorrhizae are known to differ in their
response to fertilization. The data
from this experiment suggests the feasibility of selecting mycorrhizae that
tolerate high levels of plant P. These
data may also suggest another intriguing possibility: the mycorrhizae control
mechanism in the plant may act to inhibit development of new infection rather
than inactivate the functioning mycorrhizae.
If roots are treated with highly infective inoculum, such as infected
root segments appear to be, then a more rapid, heavy colonization of the root
may develop. If spores are used as
inoculum, the time required for spore germination and infection may be greater
than that required for the plant to begin regulating the mycorrhizae.
Increased P uptake was
not the only benefit to the host by mycorrhizae. This study was not designed to quantify the mycorrhizal effects
on the uptake of other elements because K, Zn, and Ca were added to the soil in
liberal amounts to ensure they would not limit growth.
The concentrations of K, Zn, S, and Ca in plant tops are presented in
Fig. 10-13. For K, Zn, and Ca the concentrations of mycorrhizal plants were
significantly higher (P=.05) than the nonmycorrhizal plants in the cultivated
and noncultivated soil materials. The
concentrations of K, Zn, and Ca in the inoculated plants growing in the
subsoil were significantly greater than in the plants growing in sterile and
non-sterile subsoil materials. The fact
that there were no significant differences among plants growing in sterile and
non-sterile subsoil further indicates the low inoculum density in the
subsoil. The Ca data do not support
the hypothesis of resistance to Ca transport in fungi (Rhodes and Gerdemann,
1978). Yost and Fox (in press) reported
higher Ca concentrations in mycorrhizal cowpea growing in the field as compared
to nonmycorrhizal cowpea, although Vander Zaag et al. (1979) did not observe an
increase in Ca concentration in mycorrhizal cassava as compared to
nonmycorrhizal cassava which may indicate that mycorrhizal uptake of Ca is
more influenced by plant species than by an affinity of the fungi for Ca.
With respect to S, the
concentration in the inoculated plants in the subsoil was significantly higher
than in plants grown in sterile or nonsterile subsoil materials; the S concentration
in mycorrhizal plants was significantly higher than in nonmycorrhizal plants
growing in the cultivated soil material.
The reverse was true for plants growing in the noncultivated soil
material. However if the extremely
stunted plants growing in sterile soil with low P are discounted, and
comparisons are made among the plants of similar size, then S percentages were
greater in mycorrhizal plants for all soil materials. Uptake of S has been demonstrated in mycorrhizae in onions
|
|
|
|
|
|
|
|
(Gray and Gerdemann,
1973) but the significance of the effect has yet to be determined (Rhodes and
Gerdemann, 1978). In the case of Cu,
contamination of samples precluded a detailed consideration of the data. In general Cu concentrations in mycorrhizal
plants were higher as compared to nonmycorrhizal plants in the cultivated and
noncultivated soil; Cu concentrations were higher in the inoculated plants than
in plants growing in sterile or non-sterile subsoil. In all soils the effect of sterilization was significant (Table
4).
The reports in the
literature are not consistent regarding the role of mycorrhizae in the uptake
of these nutrients, although it
is generally accepted that mycorrhizae affect
the uptake of nutrients other than phosphorus. Apparent inconsistencies in the literature may in part be
related to differences among plant species and experimental conditions. The observation that mycorrhizae enhance Si
uptake in soybean and not cowpea is an example of the interaction between plant
species and mycorrhizae (Yost and Fox, in press). The conflicting reports on K uptake by mycorrhizae may also be
related to plant species (Powell, 1975; Gerdemann, 1964).
The influence of P level
in the plant may also exert effects which confound the effects of
mycorrhizae. Phosphorus fertilization
results in increased P levels in the plant which in turn may inhibit the
development of mycorrhizae. P fertilization
may also inhibit nutrient uptake due to antagonisms between the nutrient and
phosphorus; P fertilization may also enhance the uptake of a nutrient as a
result of better plant nutrition and a larger root system.
The literature concerning
the antagonisms of Zn and Cu by phosphorus have been reviewed by Olsen
(1972). When evaluating the effects of
mycorrhizae on Zn and Cu uptake two simultaneous processes should be considered:
1. the suppression of mycorrhizal uptake of other nutrients by phosphorus
because of reduced infection; and 2. P antagonistic effects
|
|
toward Zn and Cu uptake.
P fertilization can
enhance the uptake of a nutrient due to better plant nutrition and a larger
root system, and can result in a nutrient becoming 'diluted'. The concentration of a nutrient in plant
tissue will be diluted when the rate of plant growth exceeds the rate of
nutrient uptake. When evaluating the effect of mycorrhizae, the rate of plant
growth and nutrient uptake should be considered. Only recently have researchers begun to look at the rate of both
of these processes (Post and Fox, in press; Lambert et al., 1979).
Effect
of Soil
Three criteria were used
as a basis for selecting the
soils for this
experiment: 1. the soils should have similar mineralogy; 2. the soils should be
low in phosphorus; and 3. there should be suspected differences in mycorrhizal
inoculum densities. Before plants were
grown, soils were limed and fertilized according to requirements predicted by
soil analysis. Results of soil analysis
are presented in Table 1. It is now
apparent that physical properties of the soil materials should have been
determined.
During the experiment it
was evident that each soil had its own potential for growth. A comparison of growth potentials among the
soil materials is most valid when there are no differences in inoculum density.
In Fig. 14 the yield response to soil P is presented for plants growing in
sterile cultivated, noncultivated, and subsoil materials. The growth response was dependent upon the
soil as well as the soil P level.
Water movement in the
soils was another indication of differences among the soils. After the initial wetting of the soil, the
surface of the noncultivated soil dried out.
Capillary rise was not sufficient to wet the entire soil section. In the subsoil, the surface portion remained
moist throughout the experiment. The
moisture situation in the cultivated soil was intermediate between the
noncultivated and subsoil.
A small experiment was
conducted to test the hypothesis that water movement and retention was
different in the three soils. An
attempt was made to simulate the experimental conditions.
Three 30 cm plexiglass tubes (6.8 cm diameter) were cut into six 5 cm
sections and taped together to make the soil column. A known amount of soil which had passed a 1/4 in. sieve was
placed in the columns. A piece of
cheese cloth was placed over the bottom of the column to hold the soils in place. The soil materials were saturated and equilibrated
for 24 hours. The columns were then
placed upright in a dish containing water so that water was able to move up
through the soils by capillary movement.
After 24 hours the columns were removed from the dishes and allowed to
drain for 12 hours. The columns were
covered so water loss by evaporation was minimized. After 12 hours the columns were replaced in the dishes for 24
hours after which the soils were allowed to drain for 12 hours. The columns were then disassembled and the
percent water saturation by volume was determined for each section of the
column. Water percentage by volume in the first section, where soil water
tension was least, was considered to be water content at saturation. Water retention in the other sections was
calculated relative to the water content at saturation. The water retention curves are presented in
Fig. 15. The results confirm the
hypothesis that there were large differences among the soil materials. At the top of the column, the percent
saturation was approximately 25% lower in the noncultivated than in the
subsoil. The percent saturation changed
relatively little with increasing soil water tension in the subsoil, but in the
noncultivated soil it decreased markedly.
These results undoubtedly underestimate the differences which existed in
the pots in the glasshouse. These
curves were obtained under optimum conditions of wetting and drying, but in the
glasshouse evaporation and water uptake by
|
|
|
|
the plants would further
accentuate the differences.
With the soil columns the
bulk densities of the soil materials were calculated to be 0.76, 0.88, and 1.09
for the noncultivated, cultivated, and subsoil respectively. The combination of increased bulk density and
poor aeration created conditions for plant growth that were very different in
the three soil materials. The source of
these differences may not be solely attributed to bulk density. Soils containing volcanic ash materials and
high amounts of organic matter may exhibit 'hard to wet' properties. Capillary rise is not as great in soils
exhibiting such properties; and is related to the contact angle at the
water-solid interface. Where soil
surfaces are coated with volcanic ash and organic surfactants, the contact
angle can be greater than 90o, in which case the surfaces will
resist wetting. The Wahiawa soil has exhibited
these properties (Fox, personal communication), and it is possible that surface
soil materials are inherently different from subsoil materials with respect to
the ability of water to move by capillary rise.
The differences among the
soils are likely to have an effect on the diffusion of solutes in the
soil. Nye and Tinker (1977) defined
the diffusion coefficient of nonvolatile solutes as
F = D1 θ
fl dC1/dx + Fe where
D1 is the
diffusion coefficient of the solute in free solution
θ is the fraction of
soil volume occupied by solution
fl is an
impedence factor
C1 is the
concentration of solute in the soil solution
FE is the
excess flux created by surface diffusion
The water content of the
soil thus exerts a major effect upon the diffusion coefficient of a solute in
soil. With the information from the
water retention curves, we may speculate that the diffusion coefficient for
phosphorus was greatest in the subsoil, and least in the noncultivated soil.
The effect of diffusion
coefficients on the ability of plants to absorb phosphorus will be greatest
when soil P levels are low. Nonmycorrhizal plants growing in noncultivated
soil were less able to absorb phosphorus than comparable plants in cultivated
and subsoils when the soil P levels were low (Fig, 14). The threshold value of soil P for a particular
soil, that value below which the plant is unable to extract phosphorus from
the soil, may be determined by both the P diffusion coefficient in that soil as
well as the plant species. Yost and Fox
(1979) reported similar threshold values of .012 mg P/liter for
nonmycorrhizal Allium cepa, Stylosanthes, and Leuceaena
leucocephala growing in the field.
If the roots affinity for phosphorus is similar among plant species,
then the predominant factor influencing the threshold value for P absorption
may be the P diffusion coefficient.
The principle advantage
of mycorrhizae is increased P uptake. This is possible because the hyphae
extend into the soil beyond the volume of P depletion by the root. The relative advantage of mycorrhizae
should be greatest in soils where the P diffusion coefficients are least and
the volume of P depletion is the smallest. For example, in the subsoil where
the diffusion coefficient should have been relatively high, mycorrhizae may not
be as advantageous to the host as in the noncultivated soil where the diffusion
of P should be relatively less. The
concept of specific interactions between the soil and the fungi has not
received the attention it deserves.
Cooper (1975) noted that the effects of soil properties need to be
considered when evaluating plant response to mycorrhizae, including P diffusion
rates which are not reflected in extractable P values.
The critical P level for
mycotrophy may have been influenced by the P diffusion rate in the soil. In the subsoil this critical P level was
approximately .008 mg P/liter, and in the cultivated soil it was .062 mg
P/liter. Given the more similar
physical characteristics of these two soils the relative difference in their
critical P levels for mycotrophy may also reflect different inoculum
densities. In the noncultivated soil,
with a relatively high inoculum density and relatively low P diffusion
coefficient, the lower critical level for mycotrophy is unexpected. However soil structure has other effects
besides affecting water retention and diffusion rates. Root development can be impeded in soils
with high bulk densities. In the
noncultivated soil, aeration was undoubtedly more favorable for root
development relative to the cultivated and subsoils. The lower P levels for mycotrophy together with the higher
growth potential may indicate that mycorrhizae are not a substitute for a well
developed root system. The unfavorable
soil structure for root proliferatioa may have been the limiting factor in
plant growth in the subsoil material. The lack of response to inoculation in an
environment not favorable for root growth is understandable.
Another factor which may
have contributed to different growth potentials in the soils was the available
nitrogen supply. During early growth,
leaves of plants growing in sterile soils were a dark green color, while leaves
of plants in non-sterile soils were a pale green color. Differences in nitrogen nutrition were
suspected, perhaps due to the competition-free environment in sterile soil
which allowed for rapid growth of introduced Rhizobia. Other contributing factors may have been
the release of NH4+ caused by y-irradiation
(Singh and Kanehiro, 1970) and the Birch effect. After 22 days the differences in color among the plants
disappeared. By the end of the
experiment total N uptake was greater in plants in non-sterile soil than
sterile soil (Table 4). The warm moist
conditions in the pots may have promoted mineralization of organic matter
relatively more in non-sterile soils.
The differences in organic carbon in the soils (Table 1) should be
considered a factor in the different growth potentials of the soil
materials. Organic matter may not only
have contributed to the 'hard to wet' properties of the noncultivated soil
material, but also to the greater uptake of N by plants relative to plants in
the other soil materials.
Conclusions
1. A soil dilution bioassay method can detect differences in the
mycorrhizal inoculum level in soil.
2. Soil materials should be brought to standard
conditions to ensure the accuracy of the bioassay. Soil physical properties should be considered when the standard
conditions are defined. Plant material
should also be standardized.
3. The significance of differences in mycorrhizal
inoculum level is not easily interpreted.
Attention must be given to the interaction between the soil and the fungi
in order to determine the effect of inoculum level on the growth of the host
plant.
4. Two factors are involved when considering the
amount of phosphate fertilizer required to replace mycorrhizae: l. the
effectiveness of the mycorrhizal association; and 2. the P requirement of the
soil.
5. Mycorrhizae enhance the uptake of nutrients
other than phosphorus. Potassium, Zn, S, and Ca are also transported through
the hyphae. The extent to which the
nutrition of the host improves as a result of infection depends upon specific
interactions among the fungi, the host plant, and the soil.
|
|
LITERATURE CITED
1. Abbott,
K.L., and K.D. Robson. 1977. Growth stimulation of subterranean clover
with vesicular-arbuscular mycorrhizas.
Aust. J. Agric. Res., 28: 639-649.
2. Bagyaraj,
D.J., A. Manjunath, and R.B. Patil.
1979a. Occurrence of
vesicular-arbuscular mycorrhizas in some tropical aquatic plants. Trans. Brit. Mycol. Soc. 72: 164-167.
3. Bagyaraj,
D.J., A. Manjunath, and R.B. Patil.
1979b. Interaction between a
vesicular-arbuscular mycorrhiza and Rhizobium and their effects on
soybean in the field. New Phytol. 82:
141-145.
4. Baylis,
G.T.S. 1975. The magnolioid mycorrhiza and mycotrophy in root systems derived
from it. In F.E. Sanders, B.
Mosse and P.B. Tinker (eds.) Endomycorrhizas.
Academic Press, New York. pp. 373-389.
5. Bhat,
K.K.S., and P.H. Nye. 1974. Diffusion of phosphate to plant roots in
soil. III Depletion around onion roots
without root hairs. Plant and Soil 41:
383-
6. Boatman,
N., D. Paget, D.S. Hayman, and B. Mosse.
1978. Effects of systemic
fungicides on vesicular-arbuscular mycorrhizal infection and plant phosphate
uptake. Trans. Brit. Mycol. Soc. 70:
443-450.
7. Carling,
D.E., M.F. Brown and R.A. Brown.
1979a. Colonization rates and
growth responses of soybean plants infected by vesicular-arbuscular
mycorrhizal fungi. Can. J. Bot. 57:
1769-1772.
8. Carling,
D.E., M.F. Brown and R.A. Brown.
1979b. Effects of a
vesicular-arbuscular mycorrhizal fungus on nitrate reductase and nitrugenase
activities in nodulating and non-nodulating soybeans. Phytapathology 68:
1590-1596.
9. Cochrane,
W.G. 1950. Estimation of bacterial densities by means of the "most
probable number". Biometrics 6:
105-116.
10. Cooper,
K.M. 1975. Growth responses to the formation of endotrophic mycorrhizas in Solanum,
Leptospermum, and New Zealand ferns.
In F.E. Sanders, B. Mosse, and P.B. Tinker (eds)
Endomycorrhizas. Academic Press, New
York. pp. 391-407.
11. Cooper,
K.M., and P.B. Tinker. 1978. Translocation and transfer of nutrients in
vesicular-arbuscular mycorrhizas. II.
Uptake and translocation of phosphorus, zinc, and sulphur. New Phytol. 81: 43-53.
12. Cox,
G.C., and F.E. Sanders. 1974. Ultrastructure of the host-fungus interface
in a vesicular-arbuscular mycorrhiza.
New Phytol. 73: 901-912.
13. Cress,
W.A., G.O. Throneberry, and D.L. Lindsey.
1979. Kinetics of phosphorus
absorption by mycorrhizal and non-mycorrhizal tomato roots. Plant Physiology
64: 484-487.
14. Crush,
J.R. 1973. Significance of endomycorrhizas in tussock grassland in Otago,
New Zealand. New Zealand J. Bot. 11:
645-660.
15. Daft,
M.J., and A.A. El-Giahmi. 1976. Studies on nodulated and mycorrhizal
peanuts. Ann. Appl. Biol. 83: 273-276.
16. Daft,
M.J., E. Haeskaylo, and T.H. Nicolson.
1975. Arbuscular mycorrhizas in
plants colonizing coal spoils in Scotland and Pennsylvania. In F.E. Sanders, B.
Mosse, and P.B. Tinker (eds) Endoinycorrhizas. Academic Press, New York. pp. 561-580.
17. Daft,
M.J., and T.H. Nicolson. 1969. Effect of Endogone mycorrhiza on
plant growth. III. Influence of
inoculum concentration on growth and infection in tomato. New Phytol. 68: 953-961.
18. Daft,
M.J., and T.H. Nicolson. 1972. Effect of Endogone mycorrhiza on
plant growth. IV. Quantitative
relationships between the growth of the host and the development of the
endophyte in tomato and maize. New
Phytol. 71: 287-295.
19. Fox,
R.L., and E.J. Kamprath. 1970. Phosphate sorption isotherms for evaluating
the phosphate requirements of soils.
Soil Sci. Soc. Amer. Proc. 34: 902-907.
20. Fox,
R.L., R.K. Nishimoto, J.R. Thompson, and R.S. de la Pena. 1974. Comparative external phosphorus
requirements of plants growing in tropical soils. Int. Cong. Soil Sci., Trans. 10th (Moscow, Russia) 4: 232-239.
21. Gerdemann,
J.W. 1964. The effect of mycorrhizae on the growth of maize. Mycologia 56:
342-349.
22. Gerdemann,
J.W. 1968. Vesicular-arbuscular mycorrhizae and plant growth. An. Rev.
Phytopathol. 6: 397-418.
23. Gerdemann,
J.W. 1976. Endogonaceae of India: two new species. Trans. Brit. Mycol. Soc. 66: 340-343.
24. Gerdemann,
J.W., and T.H. Nicolson. 1963. Spores of mycorrhizal Endogone
species extracted from soil by wet seiving and decanting. Trans. Brit. Mycol. Soc. 46: 235-244.
25. Gerdemann,
J.W., and J.M. Trappe. 1974. The Endogonaceae in the Pacific
Northwest. Mycologia Memoir no. 5.
26. Gerdiamann,
J.W., and J.M. Trappe. 1975. Taxonomy of the Endogonaceae. In F.E. Sanders, B. Mosse, and P.B.
Tinker (eds) Endomycorrhizas. Academic
Press, New York. pp. 35-51.
27. Giovannetti,
M., and B. Mosse. In press. An evaluation of techniques for measuring
vesicular-arbuscular infection in roots.
28. Gray,
L LT., and J.W. Gerdemann. 1973. Uptake of sulphur-35 by vesicular-arbuscular
mycorrhizae. Plant and Soil 39:
687-689.
29. Greenhalgh,
F.C. 1978. Evaluation of techniques for quantitative detection of Phytophthora
cinnamomi. Soil Biol. Biochem.
10: 257-259.
30. Hall,
I.R., and P. Armstrong. 1979. Effect of vesicular-arbuscular mycorrhizas
on growth of white clover, lotus, and ryegrass in some eroded soils. N.Z. J. Agricultural Research 22: 479-484.
31. Hall,
I.R., and B.J. Fish. 1979. A key to the Endogonaceae. Trans. Brit. Mycol. Soc. 73: 261-270.
32. Hallsworth,
E.G. 1958. In E.G. Hallsworth (ed) Nutrition of legumes.
Butterworths, London. pp. 183-
33. Harley,
J.L. 1969. The biology of mycorrhiza.
2nd ed., Leonard Hill, London.
34. Hattingh,
M.J. 1975. Uptake of 32P -labeled phosphate by
endonycorrhizal roots in soil chambers.
In F.E. Sanders, B. Mosse, and F.B. Tinker (eds)
Endomycorrhizas. Academic Press, New
York. pp. 289-295.
35. Hattingh,
M.J., L.E. Gray, and J.W. Gerdemann.
1973. Uptake and translocation
of 32P-labelled phosphate to onion roots by endonycorrhizal
fungi. Soil Sci. 116: 383-387.
36. Haynan,
D.S. 1970. Endogone spore numbers in soil and vesicular-arbuscular
mycorrhiza in wheat as influenced by season and soil treatment. Trans. Brit. Mycol. Soc. 54: 53-63.
37. Hayman,
D.S. 1974. Plant growth responses to vesicular-arbuscular mycorrhiza. VI. Effect of light and temperature. New Phytol. 73: 71-80.
38. Hayman,
D.S., and G.E. Stovold. 1979. Spore populations and infectivity of
vesicular-arbuscular mycorrhizal fungi in New South Wales. Aust. J. Bot. 27: 227-233.
39. Jackson,
N.E., R.E. Franklin, and R.H. Miller.
1972. Effects of
vesicular-arbuscular mycorrhizae on growth and phosphorus content of three
agronomic crops. Soil Sci. Sod. Am. Proc.
36: 64-67.
40. Khan,
A.G. 1972. The effect of vesicular-arbuscular mycorrhizal associations on
growth of cereals. I. Effects on maize
growth. New Phytol. 71: 613-619.
41. Kleiasctimidt,
G.D., and J.W. Gerdemann. 1972. Stunting of citrus seedlings in fumigated
nursery soils related to absence of endoinycorrhiza. Phytopathology 62:
1447-1453.
42. Kruccelmann,
H.W. 1975. Effects of fertilizers, soils, soil tillage, and plant species on
the frequency of Endogone chlamydospores and mycorrhizal infection in
arable soil. In F.E. Sanders, B.
Mosse, and P.B. Tinker (eds) Endomycorrhizas.
Academic Press, New York. pp.
511-525.
43. Lambert,
D.H., D.E. Baker, and H. Cole, Jr.
1979. The role of mycorrhizae in
the interactions of phosphorus with zinc, copper, and other elements. Soil Sci. Soc. Am. J. 43: 976-980.
44. Lewis,
D.H. 1973. Concepts in fungal nutrition and the origin of biotrophy. Biol.
Rev. 48: 261-278.
45. Malajczuk,
N., G.D. Bowen, and F.C. Greenhalgh.
1978. A combined fluorescent
antibody and soil seiving technique to count chlamydospores of Phytophthora
cinnamomi in soil. Soil Biol. Biochem. 10: 437-438.
46. Mason,
D.T. 1964. A survey of numbers of Endogone spores in soil cropped
with barley, raspberry, and strawberry.
Hort. Res. 4: 98-103.
47. Menge,
J.A., D. Steirle, D.J. Bagyaraj, E.L.V. Johnson, and R.T. Leonard. 1978a.
Phosphorus concentrations in plants responsible for inhibition of
mycorrhizal infection. New Phytol. 80:
575-578.
48. Menge,
J.A., C.K. Labanauskas, E.L.V. Johnson, and R.G. Platt. 1978b.
Partial substitution of mycorrhizal fungi for phosphorus fertilization
in the greenhouse culture of citrus.
Soil Sci. Soc. Am. J. 42: 926-930.
49. McIlveen,
W.D., R.A. Spotts, and D.D. Davis.
1975. The influence of soil zinc
on nodulation, mycorrhizae, and ozone sensitivity of Pinto Bean. Phytopath. 65: 645.
50. Moorman,
T., and F. Brent Reeves. 1979. The role of mycorrhizae in revegetation
practices in the semi-arid west. II. A
bioassay to determine the effect of land disturbance on endomycorrhizal
populations. Am. J. Bot. 66: 14-18.
51. Mosse,
B. 1973a. Advances in the study of vesicular-arbuscular mycorrhiza. Ann. Rev. Phytopathol. 11: 171-196.
52. Mossa,
B. 1973b. Plant growth responses to vesicular-arbuscular mycorrhizae. IV. In soil given additional phosphate. New Phytol. 72: 127-136.
53. Mosse,
B. 1977. Plant growth responses to vesicular-arbuscular mycorrhiza. X. Responses of Stylosanthes and
maize to inoculation in unsterile soils.
New Phytol. 78: 277-288.
54. Mossia,
B. 1979. VA mycorrhiza research in tropical soils. A state of the art study prepared for the
University of Hawaii.
55. Mossia,
B., and G.D. Bowen. 1968a. A key to the recognition of some Endogone
spore types. Trans. Brit. Mycol. Soc.
51: 469-483.
56. Mosse,
B., and G.D. Bowen. 1968b. The distribution of Endogone spores
in some Australian and New Zealand soils and in an experimental field soil at
Rothamsted. Trans. Brit. Mycol. Soc.
51: 485-492.
57. Mosse,
B., and G.W. Jones. 1968. Separation of Endogone spores from
organic soil debris by differential sedimentation on gelatin columns. Trans.
Brit. Mycol. Soc. 51: 604-608.
58. Mossia,
B., C.L1. Powell, and D.S. Hayman.
1976. Plant growth response to
vesicular-arbuscular mycorrhiza. IX.
Interactions between VA mycorrhiza, rock phosphate, and symbiotic nitrogen
fixation. New Phytol. 76: 331-342.
59. Munns,
D.N. 1977. Mineral nutrition and the legume symbiosis. In R.F.W. Hardy, and A.H. Gibson
(eds) A treatise on dinitrogen fixation. John Wiley and Sons, Inc., New
York. pp. 354-391.
60. Murdoch,
C.L., J.A. Jackobs, and J.W. Gerdemann.
1967. Utilization of phosphorus
of sources of different availability by mycorrhizal and nonmycorrhizal maize. Plant and Soil 27: 329-334.
61. Nicolson,
T.H. 1960. Mycorrhiza in the Gramineae.
II. Development in different habitats, particularly sand dunes. Trans. Brit. Mycol. Soc. 43: 132-145.
62. Nicolson,
T.H. 1967. Vesicular-arbuscular mycorrhizas- a universal plant
symbiosis. Sci. Prog. Oxf. 55: 561-581.
63. Nicolson,
T.H., and J.W. Gerdemann. 1968. Mycorrhizal Endogone species.
Mycologia 60: 313-325.
64. Nye,
P.H., and P.B. Tinker. 1977. Solute movement in the soil-root
system. University of California Press,
Berkeley.
65. Ohms,
R.E. 1957. A flotation method for collecting spores of a Phyconycetous
mycorrhizal parasite from soil.
Phytopathology 47: 751-752.
66. Olsen,
S.R. 1972. Micronutrient interactions. In J.J. Mortvedt, P.M.
Giordano, and W.L. Lindsay (eds) Micronutrients in Agriculture. Soil Science Soc. Am., Inc. Madison,
Wisconsin. pp. 243-264.
67. Owusu-Bennoah,
E., and B. Mosse. In press. Plant growth responses to
vesicular-arbuscular mycorrhiza. XI
Field inoculation responses in barley, lucerne, and onion.
68. Owusu-Bennoah,
E., and A. Wild. 1979. Auto radiography of the depletion zones of
phosphate around onion roots in the presence of vesicular arbuscular
mycorrhiza. New Phytol. 82: 133-140.
69. Pearson,
V. and P.B. Tinker. 1975. Measurement of phosphorus fluxes in the
external hyphae of endomycorrhizas. In
F.E. Sanders, B. Morse and P.B. Tinker (eds.) Endomycorrhizas. pp. 277-287.
70. Peyronel,
B., B. Fassi, A. Fontana and J. Trappe.
1969. Terminology of Mycorrhizae. Mycologia 61: 410-411.
71. Phillips,
J.M., and D.S. Hayman. 1970. Improved procedures for clearing roots and
staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid
assessment of infection. Trans. Br.
Mycol. Soc., 55: 158-161.
72. Porter,
W.M. 1979. The 'most probable number' method for enumerating infectioe
proagules of vesicular-arbuscular mycorrhizal fungi in soil. Aust. J. Soil Res.
17: 515-519.
73. Powell,
C. Ll. 1975. Potassium uptake by endotrophic mycorrhizas. In F.E. Sanders, B. Mosse, and P.B.
Tinker (eds) Endomycorrhizas. Academic
Press, New York. pp. 461-468.
74. Powell,
C. Ll. 1976a. Development of mycorrhizal infection from Endogone spores
and infected root segments. Trans.
Brit. Mycol. Soc. 66: 429-435.
75. Powell,
C. Ll. 1976b. Mycorrhizal fungi stimulate clover growth in New Zealand hill
country soils. Nature, London 264:
436-438.
76. Powell,
C. Ll. 1979a. Inoculation of white clover and ryegrass seed with mycorrhizal
fungi. New Phytol. 83: 81-85.
77. Powell,
C. Ll. 1979b. Spread of mycorrhizal fungi through soil. N.Z. Journal of
Agricultural Research 22: 355-359.
78. Ratnayake,
M., R.T. Leonard, and J.A. Menge.
1978. Root exudation in relation
to supply of phosphorus and its possible relevance to mycorrhizal
formation. New Phytol. 81: 543-552.
79. Read,
D.J., H.K. Koucheki, and J.Hodgson.
1976. Vesicular-arbuscular
mycorrhiza in natural vegetation systems.
New Phytol 77: 641-653.
80. Redhead,
J.F. 1977. Endotrophic mycorrhizas in Nigeria. Species of Endogonaceae and their distribution. Trans. Brit. Mycol. Soc. 69: 275-280.
81. Reeves,
F., D. Wagner, T. Moorman, and J. Kiel.
1979. The role of
endomyrcorrhizae in revegetation practices in the semi-arid west. I. A comparison of incidence of mycorrhizae
in severely disturbed vs. natural environments. Am. J. Bot. 66: 6-13.
82. Rhodes,
L.H. 1979. P uptake by vesicular-arbuscular mycorrhizae. In Abstracts from the Fourth North
American Conference on Mycorrhiza. Colorado State University Fort Collins,
Colorado.
83. Rhodes,
L.H., and J.W. Gerdemann. 1978a. Translocation of calcium and phosphate by
external hyphae of vesicular-arbuscular mycorrhizae. Soil Science 126: 125-126.
84. Rhodes,
L.H., and J.W. Gerdemann. 1978b. Influence of phosphorus nutrition on sulfur
uptake by vesicular-arbuscular mycorrhizae of onion, Soil Bio. Biochem. 10:
361-364.
85. Ross,
J.P. 1979. Factors affecting sporulation of endomycorrhizal fungi. In Abstracts from the Fourth North
American Conference on Mycorrhiza.
Colorado State University Fort Collins, Colorado.
86. Sanders,
F.E. 1975. The effect of foliar applied phosphate on the mycorrhizal
infections of onion roots. In
F.E. Sanders, B. Mosse, and F.B. Tinker (eds) Endomycorrhizas. Academic Press, New York. p. 261-
87. Sanders,
F.E. 1976. What directions should be taken in future research at IITA on the
role of mycorrhizas in crop nutrition?
A report prepared for IITA.
88. Sanders,
F.E., and P.B. Tinker. 1971. Mechanism of absorption of phosphate from
soil by Endogone mycorrhizas.
Nature 233: 278-279.
89. Singh,
B.R., and Y. Kanehiro. 1970. Effects of gamma irradiation on the
available nitrogen status of soils. J.
Sci. Fd Agric. 21: 61-64.
90. Smith,
G.W., and H.D. Skipper. 1979. Comparison of methods to extract spores of
vesicular-arbuscular mycorrhizal fungi.
Soil Sci. Soc. Am. J. 43: 722-725.
91. Sondergaard,
M., and S. Laegaard. 1977. Vesicular-arbuscular mycorrhiza in some
aquatic vascular plants. Nature 268:
232-233.
92. Sparling,
G.P., and P.B. Tinker. 1978. Mycorrhizal infection in Pennine
grassland. II. Effects of mycorrhizal
infection on the growth of some upland grasses on gamma irradiated soils. J. Applied Ecology 15: 943-950.
93. Stanford,
G., and J.D. Dement. 1957. A method for measuring short-term nutrient
absorption by plants. I.
Phosphorus. Soil Sci. Soc. Am. Proc.
21: 612-617.
94. Strzemska,
J. 1974. The occurrence and intensity of mycorrhizas in cultivated
plants. Proc. 10th Int. Cong. Soil Sci.
Moscow 3: 81-86.
95. Sutton,
J.C., and J.L. Barron. 1972. Population dynamics of Endogone spores
in soil. Can. J. Bot. 50: 1909-1914.
96. Thaxter,
R. 1922. A revision of the Endogoneae.
Proc. Am. Acad. Arts Sci. 57: 291-351.
97. Tinker,
P.B. 1975a. Effects of vesicular-arbuscular mycorrhizas on higher
plants. Symp. Soc. Exp. Biol. 29:
325-349.
98. Tinker,
P.B. 1975b. Soil chemistry of phosphorus and mycorrhizal effects on plant
growth. In F.E. Sanders, B. Mosse,
and P.B. Tinker (eds) Endomycorrhizas.
Academic Press, New York. pp. 353-371.
99. Tsao,
P.H. 1960. A serial dilution end point method for estimating disease
potential of citrus Phytophthoras in soil. Phytopathology 50: 717-724.
100. Vander
Zaag, P., R.L. Fox, R.S. De la Pena, and R.S. Yost. 1979. P nutrition of
cassava, including mycorrhizal effects on P, K, S, Zn, and Ca uptake. Field Crops Res. 2: 253-263.
101. Waidyanatha,
U.P. De S., N. Yogaratnam, and W.A. Ariyaratne. 1979. Mycorrhizal
infection on growth and nitrogen fixation of Pueraria and Stylosanthes
and uptake of phosphorus from two rock phosphates. New Phytol. 82: 147-152.
102. Walker,
C. 1979. The mycorrhizast and the herbarium: The preservation of
specimens from VA mycorrhizal studies. In
Abstracts from the Fourth North American Conference on Mycorrhiza. Colorado State University, Fort Collins,
Colorado.
103. Walkley,
A. 1935. An examination of methods for
determining organic carbon and nitrogen in soils. J. Agr. Sci. 25: 598-609.
104. Wiltelm,
S. 1966. Chemical treatments and inoculum potential of soil. Ann. Rev.
Phytopath. 4: 53-78.
105. Yost,
R.S., and R.L. Fox. 1979. Contribution
of Mycorrhiza to the P nutrition of crops growing on an Oxisol. Agron. J. 71: 903-908.
106. Yost,
R.S., and R.L. Fox. In press. Influence of Mycorrhizae on the mineral
contents of cowpea and soybean grown in an Oxisol.