Tolerances to Soil Acidity Factors
Among Rhizobia
By
HAROLD HAUN KEYSER
B.S. (University of California, Davis)
1970
M.S. (University of California, Davis)
1973
DISSERTATION
Submitted in partial satisfaction of the
requirements for the degree of
DOCTOR
OF PHILOSOPHY
in
Soil Science
in
the
GRADUATE
DIVISION
of
the
UNIVERSITY
OF CALIFORNIA
DAVIS

Committee
in Charge
Deposited in the University Library...................................
Date
Librarian
TABLE
OF CONTENTS
Page
ACKNOWLEDGMENTS 3
ABSTRACT 4
INTRODUCTION AND LITERATURE REVIEW 8
SECTION
I. Effects of Acidity, Aluminum‑and
Phosphate
on Growth of Rhizobia 23
SECTION
II. Effects of Calcium and
Manganese in Combination
with. Other Soil Acidity Factors
on Growth of
Rhizobia 43
SECTION III.
Adaptation to Aluminum by Rhizobia 63
SECTION
IV. Relationship Between
Rhizobial Tolerances in
Pure Culture and Symbiotic Performance
in
Acid Soils 68
APPENDIX.
Rhizobial Strains 101
BIBLIOGRAPHY 104
ACKNOWLEDGMENTS
The
author wishes to thank Drs. D. Munns, R. Valentine and C. Delwiche for their
advice, discussion and critique of this thesis. Special thanks is given to Dr.
Munns for his patience, criticism, encouragement and general inspiration.
Thanks
are given to Drs: D. Weber, J. Burton, V. Reyes, T. Wacek and D. Date for
supplying strains of rhizobia.
Also,
thanks are extended to Julia Hohenberg, David Lauter, Peter Vonich, Vince
Fogle, Mahgoub Zaroug, Kim Clarkin, Tim Righetti and
for valuable discussion and
assistance in technical, analytical and statistical matters, to Pete Pankratz
for the line drawings, and to Cheryl Wood for typing the thesis.
I
am deeply grateful to my parents for their encouragement and assistance in
numerous ways, and especially so to my wife Anne and daughter Arica for their
understanding and patience with my temperament during the writing of this
thesis.
ABSTRACT
Tolerance
of low pH (4.5), low phosphate (5‑10 µM), and high Al (50 µM) was
assessed in 10 strains of cowpea rhizobia by detailed growth studies based on
viable counts in defined liquid glutamate/mannitol media, and in 65 strains of
cowpea rhizobia and R. japonicum by a rapid method based on
attainment of turbidity from a small inoculum.
Strains varied in response.
Aluminum was the most severe stress.
Low
P (as compared with 1000 µM) limited total attainable population density to 5 x
107 cells/ml, and slowed the growth of some strains. Acidity increased lag time or slowed growth
of most strains, and stopped growth of about 30% of them. Tolerance of acidity did not necessarily
entail tolerance of the Al that would normally be associated with soil pH
4.5. Aluminum (50 µM) increased lag or
slowed growth of almost all the strains tolerant of low pH, and virtually
stopped growth of half of them.
Despite
weak buffering, pH was adequately controlled by working at low population
density, beginning with inoculation at 103 cells/ml, because the pH
did not rise by 0.1 unit until cultures had become almost visibly turbid at 107
cells/ml. Thus the rhizobia had to make
1000‑fold growth under the stress before they could significantly raise
pH and precipitate Al. Tolerant strains
therefore had a real tolerance that might be expressed in buffered soil
environments. A valid rapid screening
can be based on ability to attain visible turbidity in culture under acid or Al‑stress,
so long as inoculum level is small (<< 105 cells/ml).
Growth
studios were done in defined liquid media to assess likely effects of Mn
toxicity and Ca deficiency associated with soil acidity.
The study included 23
strains of cowpea rhizobia previously found
capable of growth at pH 4.5
and 10 strains of R. japonicum tolerant of pH 4.8. The low level of Ca (50 µM) represented the
extreme low range in soil solutions, and the high level of Mn (200
µM) has been found toxic to legume hosts of the strains tested.
In
a detailed growth study of 3 cowpea strains at pH 4.6, low P (10 µM) limited
maximum viable cell density in all 3 strains.
Low Ca limited it in 1 strain.
High Mn reduced growth rates of 2 strains.
A
rapid screening method based on attainment of turbidity from a small inoculum
was applied to the cowpea rhizobia at pH 4.5 and soybean rhizobia at pH
4.8. High Mn and low Ca slowed growth
of only 3 strains. Neither was as
severe a stress as 50 µM Al, simultaneously observed and previously
reported. All strains tolerant of Al
were tolerant of Mn and low Ca.
Possible
amelioration of Al toxicity by Ca was tested in 3 cowpea strains, by a
factorial experiment with 3 Ca levels (50‑1000 µM) and 4 Al levels (0‑100
µM), at pH 4.5 in liquid media. Calcium had statistically significant pretective effect against
Al in two strains, but the effects were small and probably of no biological or
practical significance.
In
acid soils, Al toxicity and acidity itself are probably more important limiters
of rhizobal growth than Mn toxicity and Ca deficiency.
As
a preliminary investigation into rhizobial adaptation to acid‑Al stress,
ten strains were introduced, each at widely varying inoculum levels, into pH
4.5‑5 µM P ‑ 50 µM Al broth medium. Five strains attained relatively high population levels in 17
days from very few initial cells, three strains needed higher initial numbers to make net growth, and then had slower growth rates than the first group, and one strain showed
death of cells at all inoculum levels.
It was concluded that the mechanism of Al‑adaptation was not spontaneous mutation, being due either to the existence of
genetic variants within a population or the phenotypic adaptation of similar
cells.
In
greenhouse pot trials, 25 strains of rhizobia were tested on 3 host varieties
of Vigna unguiculata (cowpea) on 2 acid soils. The symbiotic effectiveness of strains at pH
4.6 and 6.0‑6.2 was compared with their known tolerances to acidity and Al in defined laboratory
media. One soil was naturally acid at
pH 4.6, and the other was acidified to this pH with Al2(SO4)3. Large differences were found between strains
in ability to produce good plant growth at low pH. The general effects of acidity on both soils was reduced plant
growth associated with decreased nodule abundance and mass, as compared with
that at pH 6.0‑6.2. Symbiotic
growth at low Ph in the soil which received Al2(SO4)3
was more inhibited than in the soil with the same pH but lower available
Al. Most importantly, the strains which gave the best yields in both soils at pH 4.6 were all tolerant of acid
and Al in laboratory media. The
prescreening of strains in various acid broth media appears to be a promising,
worthwhile procedure in selecting tolerant candidate strains for use in acid
soils.
INTRODUCTION
AND LITERATURE REVIEW
This
thesis reports on the effects of certain soil acidity factors upon the growth
and symbiotic performance of slow growing strains of the symbiotic, nitrogen‑fixing
bacteria Rhizobium.
The
natural, nonsymbiotic habitat of rhizobia is the soil, and the immediate
environment is that of the soil solution.
In an acid soil, besides the high hydrogen ion activity in solution,
there are several mineral elements whose pH‑dependent solubility and
reaction with the soil matrix can produce relative toxic or deficient growth
effects on higher plants (Pearson and Adams 1967; Pearson 1975; Kamprath
1972). Presumably these same factors
could be stressful in rhizobia also, though much less information is available on this (Munns 1977b; 1978;
Rerkasem 1977; Carvalho 1978).
The
most commonly reported mineral elements producing these effects in acid soils
are calcium, magnesium, phosphorus, and molybdenum in the deficiency category,
and aluminum, manganese, and hydrogen‑ion in the toxicity category
(Pearson and Adams 1967; Pearson 1975; Munns 1978). Though any one of
these soil acidity factors alone can produce a biological stress, in many acid
soils several of them occur together, and would then interact in their effects
on the plant or microbe (Munns 1977b; Pearson 1975).
The
factors chosen for study of their effects on rhizobia reported in this thesis
are calcium, phosphorus, manganese, and aluminum, at low pH in pure culture
studies, as well as the effects of two acid‑infertile soils.
The Bacterium
The
Eubacteriales genus Rhizobium is characterized as gram‑negative,
nonspore‑forming, short rods,
about 0.5 to 0.9 by 1.2 to 3.0 µM, though it is commonly pleomorphic under
adverse stress condtions (Jordon and Allen 1975; Vincent 1962). Members of the genus characteristically
invade roots of legumes and induce root nodules to form, and the different
strains exhibit host range affinities.
The nodule bacteroids are involved in fixing molecular nitrogen into combined forms utilizable by the host
plant (Vincent 1970).
The
bacteria are chemoorganotrophs with a respiratory metabolism. They are aerobic, and often able to
produce excellent growth under 02 tensions less than
0.01 atmosphere. The optima for
temperature and pH is 25‑25°C and 5.0‑8.5 respectively. Most satisfactory growth is provided by
media containing yeast or other plant
extracts, and yeast extract‑mineral salts media containing mannitol or
glucose are among the most conventional (Jordan and Allen 1975).
The
taxonomic division of Rhizobium (Jordon and Allen 1975) is based upon
host affinity for nodulation, and two major groups are recognized:
Group l ‑
2 to 6 peritrichous flagella, rapid growth on yeast extract media.
R. leguminosarum R.
trifolii
R. phaseoli R. meliloti
Group 2 ‑ polar or subpolar flagellum, slow
growth on yeast extract media.
R. japonicum R. lupins
Another
large group of mostly slow growing strains is recognized by many, referred to
as the "cowpea rhizobia" or the "cowpea miscellany", as
well as a smaller, odd group termed the lotus rhizobia (Vincent 1970, 1974).
The
representative preferred hosts for all of the species are compiled elsewhere
(Jordon and Allen 1975; Vincent 1970, 1974).
As
Vincent (1974) points out, the
distinction between the fast and slow growing rhizobia is well established on
the basis of growth characteristics, acid production in synthetic media,
utilizable carbon source, complement of glycolytic enzymes and internal
antigens, as well as evidence from numerical taxonomy.
A Review of the Pertinent
Literature
pH
Of
all the soil acidity factors, the effects of pH per se on rhizobia has been the
most thoroughly researched. Early work
on the response of several strains to low pH liquid media revealed that the
different species of rhizobia had different critical pH for growth, R. meliloti
being the most acid sensitive with a critical pH of 4.9, and the slow growing R.
japonicum and R. lupini were the most acid tolerant,
having critical pH of 3.3 and 3.15 respectively (Fred and Davenport 1918).
From
a study of 8 strains of R. japonicum, Wright (1925) found the
critical pH range in liquid media to be 4.1 to 4.5, though 5 of the 8 strains showed net viability decrease at pH
less than 4.4 within 2 weeks.
Fairly
good agreement with these values was found by Bryan (19.23) who introduced
strains of different species into acid soils and found the critical pH for
their recovery to be 5.0 for R. meliloti, 4.5‑4.7 for R.
trifolii, and 3.5‑3.9 for R. japonicum. Comparing 2
fast growing species for acid tolerance in liquid media, Jensen (1942)
confirmed that R. trifolii is more tolerant than R. meliloti,
but both were inhibited below pH 5.
The most
extensive investigation of the sensitivity to pH among rhizobia was conducted
by Graham and Parker (1964) who tested 79 strains in liquid medium and found
the general order of tolerance to low pH to be R. meliloti other fast growers slow growers. Of great significance was the demonstration of
differential strain tolerances within each species. Their findings, as well as more recent reports (Graham
and Hubbell 1975; Broughton et al. 1975) puts the critical pH range for the
more tolerant slow growers at 4.3‑4.9, a range more conservative than the
earlier studies.
The
results from several studies on the effects of acidity as tested in soil give
good agreement with artificial media tests concerning the more important
observations; the critical pH ranges, the relative tolerances between rhizobium
species, and within species the existence of strain to strain differences. Studies on isolates of the slow grower R.
japonicum from Iowa soils show that some strains (sero‑groups) are
indeed acid tolerant with a lower pH limit for growth of 4.0, while there exist
large sero-group differences in adaptation to soils in the 7.5‑8.0 range
(Damirgi et al. 1967; Ham et al. 1971).
Among the fast growers, R. trifolii is found to persist
better in soils at a lower pH than R. meliloti, though they both
exhibit increased population levels in more neutral soils (Jones 1966; Jensen
1969; Munns 1965a; Nutman and Ross 1969; Peterson and Gooding 1941). Even the acid sensitive R. meliloti
is found to have significant strain differences to survival in acid soil (Munns
1965a; Robson and Loneragan 1970a) so that these authors noted the possible
benefits accruing from selection of R. meliloti strains for their
performance in acid soils. Also, Lie
(1971) found the same strain differentiations occurring at low pH among R.
leguminsoraum, and these differences were not predictable from the
strain's symbiotic performance at a neutral pH. Related studies of the cowpea type rhizobia show that in this
acid tolerant group there also exists strain variation (Norris 1973; Rerkasem
1977).
The
general association of the most acid tolerant rhizobia with the slow growth
habit was a fundamental tenet in Norris' hypothesis (Norris 1965) that the
prototype Rhizobium was a cowpea type organism that evolved in the wet,
acid soils of the tropics, along with the tropical legumes, this group also supposedly
being the more primitive line of the host plant family. Norris associated a broad host‑range
compatibility with this primitive condition of the symbiosis. From tests on over 700 strains, he
emphasized that the slow growing strains produced a net alkaline reaction on
agar medium and that they were associated with, or isolated from, the most acid
tolerant hosts, as well as themselves being the most acid tolerant group of rhizobia, and that there would be an
ecological advantage for an alkaline producing strain over an acid producing
strain (the fast grower) in an acid soil.
Though there are reports giving support for some aspects of his
hypothesis (Norris 1973; Brockwell et al. 1966; Rerkasem 1977), Parker (1968)
has challenged many theoretical aspects of the hypothesis, and specifically
Parker (1971) was able to demonstrate that the relative pH alteration of media
is very dependent on its composition and that it would be unreliable to assume
that the rhizobia produce similar alterations of pH in the buffered soil
itself. Neither slow nor fast growing
rhizobia were able to alter the pH of an unmodified soil extract solution,
despite good growth, thereby showing no relationship with their respective
abilities to change the pH in conventional media. Also, Munns et al. (1978) in a screening of 40 strains of the
cowpea miscellany found only a weak association between alkali production in
media and symbiotic acid tolerance, alkali production being a very imprecise
indicator of acid tolerance. There appears
to be little disagreement over the relative acid tolerances of the species of Rhizobium,
but there is not enough information available concerning the evolution of the
symbiosis or the bacterial mechanisms of acid tolerance to put these together
into a unifying concept that relates them all.
Several
soil studies have shown that applied rhizobia fail to persist or colonize in
acid soils (Vincent and Waters 1954a & b; Vincent 1958;
Robson and Loneragan 1970a; Mulder et al. 1966). Related research has shown that large innoculum levels can
somewhat replace the beneficial effect of timing (Spencer 1950; Vincent and
Waters 1954b; Mulder and Van Veen 1960; Robson and Loneragan 1970a; Rerkasem
1977), this being demonstrated for R. trifolii, R. meliloti,
and the cowpea miscellany.
There
are reports in the literature that poorly effective, indigenous, strains of
rhizobia are more common in acid soils, with the effectiveness increasing in
neutral soils (Holding and King 1963; Jones 1966; Jones and Burrows 1969). Also, Van Schreven (1972) demonstrated
differences in strain response to the effect of subculturing at low pH on agar
media for 300 days on this subsequent effectiveness. Holding and King (1963) suggested that the more ineffective
strains found at low pH may be more tolerant of elements such as Mn and Al as
compared with more effective strains.
While all the above work on the relationship between ineffectiveness and
soil acidity has been done with R. trifolii, Munns et al. (1978)
working with the cowpea miscellany group found no trend for the symbiotic acid
tolerant strains to be reduced in comparative effectiveness.
Abundant
literature exists on the effects of acidity on different aspects of the
symbiosis as separate from effects on the Rhizobium per se (Vincent
1965; Andrew 1976a; Jensen 1943, 1947; Lowther and Longeragan 1970; Munns
1968), and the reader is directed to excellent, current review on this subject
(Andrew 1978; Munns 1977a & b, 1978; Lie 1971, 1974; Rerkasem 1977;
Carvalho 1978).
Calcium
The
requirement for Ca as an essential nutrient for rhizobia is quite small, as
determined in liquid media (Loneragan and Dowling 1958; Norris 1959; Bergersen
1961; Vincent 1962). Vincent (1962)
shows that the Ca requirement is about 25 µM for normal growth in liquid
culture. In that study he also found no
effect of pH down to 5.5 on the response to Ca levels. Humphrey and Vincent (1962) have shown that the Ca is an essential component of the cell wall fraction of the Rhizobium.
While
Bergersen (1961) did find differences between strains in growth characteristics
in low Ca broth, Norris (1959) reported differences in Ca requirement between
fast and slow growing rhizobia when studied
in electrodialyzed clay systems.
Rerkasem (1977) points out that the differential Ca requirements noted
by Norris may only reflect a difference in ability to obtain or retain calcium
in those conditions. Nonetheless, she
reports that many fast growers responded to increased Ca at pH 3.9, while many
of the slow growers studied showed no such response above pH 2.8. Also, Lowther and Longeragan (1970) obtained
a positive response to Ca for the fast growing R. trifolii in the
rhizosphere of subclover in solution culture at pH 5.0.
Reports are scant on the effects of neutral
calcium salts on rhizobia in soil.
Albrecht and Davis (1929) reported better survival of rhizobia in soil,
as assessed by nodulation on a second crop of soybeans, when CaCl2
was supplied. Anderson and Maye (1952)
suggested that the gradual improvement of modulation on an acid soil might be
attributable to the Ca or P applied in the basal superphosphate fertilizer
used. However, recent work of Rerkasem
(1977) showed no effect of CaS04 addition on growth or survival of
either a fast or slow growing cowpea rhizobium in soil at pH 4.5.
The
low Ca requirement for rhizobia appears below that needed for adequate nodule
initiation and function, and general symbiotic growth, those aspects of the
slymbiosis as affected by Ca being reviewed elsewhere (Munns 1977a & b,
1978; Andrew 1978; Robson 1978). It
appears then that a deficiency of Ca in acid soil would probably have
deleterious effects on modulation and legume growth before it would limit
rhizobial growth and survival, but there is insufficient data on important
rhizosphere effects to make this a definite conclusion (Munns 1977b).
Phosphorus
Phosphorus
as a soil acidity factor is important because it is often limiting to plat
growth, being available at low levels in acid soils (Hsu 1965; Fox 1978;
Kamprath 1973; Sanchez 1976). Though it
is accepted as an essential nutrient for rhizobia, the quantitative requirement
for P in the free living state has not been reported. Luria (1960) reports the
range of dry weight P content in bacteria to be 2 to 6 percent.
Truesdell
(1917) added 3 levels of phosphate, as the Na, K or Ca salt to sterilized soil,
and between the 2 highest levels obtained large growth responses with
introduced R. meliloti.
Kamata (1962) reported that some strains of R. japonicum
did not nodulate P‑deficient soybean roots, and this was correlated to
the relative P response of the two strains in culture media. Clearly the quantitative response of
rhizobia to P in synthetic media and soil needs further elucidation.
In
the legume‑Rhizobium symbiosis, phosphorus is required in the ATP
functioning in the nitrogen fixation process (Bergersen 1971). There are
reports of added P improving nodulation, but no clear indication that these are
not simply due to improved host growth (Munns 1977b; Carvalho 1978; Fox 1978; Andrew
and Jones 1978). Manganese
In
acid soils, manganese toxicity can be a stress factor to plant growth (Pearson
1975; Pearson and Adams 1967; Sanchez 1976).
While it is difficult to find data on soil solution concentrations
associated with manganese toxicity, Asher and Edwards (1978) say that a value
of about 100 µM is sufficient to cause toxicity to leguminous plants, and this is in fair agreement with
solution levels found in acid, Mn‑toxic
soils (Morris 1948). Studies on the
effects of Mn on nonsymbiotic legumes in solution culture show that most
species are reduced in growth at manganese concentrations between
100 and 200 µM (Morris and Pierre
1949; Andrew and Regarty 1969).
Wilson
and Reisenauer (1970) demonstrated a lower optimum growth range for Mn by
rhizobia to be between 0.1 and 1 µM, and found no depressive effect of the highest level, 10 µM.
Two
strains of R. phaseoli have been reported to differ in tolerance
to Mn at very high levels, up to 400
ppm in culture media (Dobereiner 1966).
However, when these same strains were used as inocula in acid soil with
manganese added to inhibitory level, they displayed different effects on
reduction in nodule numbers or in nitrogen fixation per unit nodule weight,
indicating Mn toxicity effects are different for bacterial growth than for the
symbiotic relationships. The author
also stressed the importance of selecting rhizobia for their tolerance to soil
acidity when selecting inocula for such problem soils.
Masterson
(1968) found that R. trifolii isolates from mineral acid soils
were less effective on their host than isolates from acid peat soils, implying
that the more tolerant strains were less effective. He suggested the peat may provide some protection from high
levels of Mn, Al or Fe found at low pH.
He also reported data of Sherwood where culturing strains of R. trifolii
on media with 800 to 1500 ppm Mn showed little effect, and in some cases raised
the effectiveness. Related to these
observations are those of Holding and Lower (1971) showing that high levels of
Mn (16 mm) in continuous culture can reduce the symbiotic effectiveness of R.
trifolii. The effect was
transient though, in that good effectiveness was recoverable upon transfer to
media low in Mn.
In
studying the effects of Mn on nitrogen fixation in Beijerinckia and
Azotobacter, Becking (1961) found differential strain tolerance to 20 ppm Mn
for Azotobacter, and the Beijerinckia strain was little affected by 40 ppm,
except at a low pH of 3.0‑3.2.
The
effects of Mn on the symbiotic and nonsymbiotic legume are reported elsewhere
(Andrew and Regarty 1969; Robson and Loneragan 1970b; Vose and Jones 1963;
Morris and Pierre 1949; Souto and Dobereiner 1969; Dobereiner and Aronovich
1965; Dobereiner et al. 1965), as well as in recent reviews (Andrew 1978; 1976;
Munns 1978). A survey of this
literature reveals that Mn as a soil acidity factor can be quite limiting to
symbiotic legumes, and that the effects could be more clearly understood with
more knowledge of the effects of Mn at low pH on the rhizobia.
Aluminum
The
high levels of aluminum that occur in soil solutions at low pH can be quite
toxic to plant growth, and its abundance on the exchange sites of acid mineral
soils make it the most important soil acidity factor (Kamprath 1972; Foy 1976;
Pearson 1975; Andrew 1978). The few reports dealing with the effects of Al on
rhizobia and other, microorganisms are reviewed here.
The
important effects of Al on the functioning of the symbiotic and nonsymbiotic
legume are detailed elsewhere (Munns
1965b; Carvalho 1978; Andrew et al. 1973; Foy and Brown
1964; Foy et al. 1969), and discussed in recent reviews (Asher
and Edwards 1978; Andrew 1978). The
work of Carvalho (1978) alone shows clearly that Al is more detrimental to
symbiotic versus nonsymbiotic growth for Stylosanthes, though there was
a range of tolerance among the 6 species tested. And since only 1 rhizobial strain was used, it merits further
research to see if strains with superior Al-tolerance could be identified and
compared with less tolerant strains in their symbiotic performance with a
tolerant host.
Much
of the work on Al suffers from lack of control of Al concentration and pH
(Munns 1965b; Andrew 1978). In studies
concerning the levels of P and Al available in nutrient solutions at low pH,
Munns (1965b) reported that if P was kept below 50 µM, (1.5 ppm) at pH 4, or below 10 PM at pH 4.5, then Al concentrations on the order of 100 µM (2.7 ppm) could be
maintained without reaction with phosphate.
Andrew (1978) pointed out that many reports on the effects of Al on
plants have utilized treatments that greatly exceeded the solubility
limitations in the pH‑P‑Al system, and notes that even at low pH,
Al concentrations in soil solutions rarely exceed 4 ppm.
Early
research by Whiting (1923) demonstrated that rhizobia were not inhibited in
their growth by addition of insoluble Al salts, but were inhibited by Al salts
which lowered the pH of the media, though no data was given.
The
existence of differential species tolerance to Al among Azotobacter has been
shown by Katznelson (1940). In liquid
media, with a pH range from 4 to 7, and with Al up to 100 ppm, A. indicum
was much more tolerant than A. chroccoccum, the former not being
seriously inhibited except at pH 4.0 with 100 ppm Al. The effects of Al at low pH were clear, if not the actual
aluminum levels; many of the treatments probably exceeded the solubility of
gibbsite.
In
a similar study, Becking (1961) investigated effects of Al on the nitrogen
fixation (growth) of 2 strains of Beijerinckia and one of Azotobacter in liquid
media. The results showed the superior
tolerance of Beijerinckia over Azotobacter, although the Al standard solution
was neutralized before addition, and the organisms lowered the pH of the
medium.
Chlorella pyrenoidosa,
a green alga, has been reported by Foy and Gerloss (1972) to be quite tolerant
of aluminum, and a strain having yet greater tolerance was obtained by
adaptation to increasing Al stress.
Again, while the relative effects of A1 were clear, the quantitative levels
of Al reported are open to question because some of the treatments would have
exceeded the solubility of variscite.
The
only direct studies of effectively controlled Al level on rhizobia relate to
survival, not growth rate. Rerkasem
(1977) has shown that a slow growing strain of cowpea rhizobia that was little
affected by introduction to soil with pH 4.5 displayed a decrease in viability
when in the same soil acidified to pH
3.9‑4.1 with aluminum sulphate, while a fast cowpea strain was inhibited by pH 4.5 alone. The presence of the
rhizosphere environment of the host root enabled both strains to better survive
in acid soil. Also, in short term tests
on retention of viability (20 minutes) over a wide pH range, she also found
that many slow growers were unaffected by low pH or high Al, while
the fast growers were inhibited by the acidity itself, with no extra
detrimental effect of the Al. The
addition of 1 MM Al2(SO4)3
caused all 12 strains of the fast growers to flocculate, whereas none of
the 12 slow growers were flocculated, the difference between the two
groups probably reflecting some basic characteristic as cell surface
properties.
A
recent study by Carvalho (1978) investigated the effects of Al on the symbioses
of 6 Stylosanthes species with the slow growing, cowpea strain C8756. In looking at the effect of Al on the
microbial component of the system, she compared the viability of the strain in
nutrient solutions at pH 4.5 and with none, 25 or 100 µM Al and found no
differences, though all treatments displayed a slow decrease in population
density through 8 days.
While
the studies of Rerkasem and Carvalho show that some rhizobia can survive at
high levels of Al, there is insufficient evidence to establish Al‑tolerance
in rhizobia as distinct from acid‑tolerance, and no data concerning
effects on their growth rate.
SECTION
1
EFFECTS
OF ACIDITY, ALUMINUM AND
PHOSPHATE
ON GROWTH OF RHIZOBIA
INTRODUCTION
Effects
of acidity on species of Rhizobium are well documented. Pure culture
studies (Fred and Davenport 1918; Wright 1925; Jensen 1942; Graham and Parker
1964) have shown the critical low pH range for growth is from about 4.0 to 6.0,
with the slower growing R. japonicum R. lupini and
cowpea miscellany being in general more acid tolerant than the others, and R.
meliloti being the most acid sensitive.
These observations on relative tolerance of the species agree with
studies in acid soils (Bryan 1923; Damirgi et al. 1967; Jensen 1969; Nutman and
Ross 1969; Norris 1973; Rerkasem 1977).
Also within each species, important strain to strain variation has been
demonstrated (Graham and Parker 1964; Munns 1965a; Damirgi et al.
1967; Ham et al. 7.971; Lie 1971; Norris 1973; Rerkasem 1977).
Besides
low pH per se, acid mineral soils have low levels of phosphorus and high levels
of aluminum (Hsu 1965; Kamprath 1973; Pearson 1975; Probert 1978). There has been little research on the
effects of these two soil factors on rhizobia.
The reports on effects of P on rhizobia are limited to an early study
showing positive growth response to P additions in soil (Truesdell 1917), a report relating inability to R.
japonicum strains to nodulate P‑deficient soybeans with their
relative response to P in culture media (Kamata 1962), and a report
demonstrating that three strains of rhizobia were better than two other common
bacteria at taking up P from very low concentrations in solution (Werner and
Berghauser 1976). Concerning Al,
Whiting (1923) stated that Al salts inhibited rhizobial growth only when they
lowered the pH of the culture media.
Recent studies (Rerkasem 1977; Carvalho 1978) have shown that some
rhizobia can indeed survive high Al concentrations at low pH in both solution
media and soil. However, there is still
insufficient evidence to establish Al‑tolerance in rhizobia as distinct
from tolerance of low pH. There are no
data concerning effects of Al on rhizobial growth rate.
The
objectives of this investigation were (i) to determine the effects of low P and
high Al on the survival and growth rate of some rhizobia at low pH, (ii) to
examine the relationship between acid‑tolerance and Al-tolerance, and
(iii) to rate the probable importance of the three stresses (acid, P and Al)
according to their inhibitory effects on rhizobial growth.
MATERIAL
AND METHODS
Rhizobia
The
65 strains of rhizobia were obtained from three sources: University of Hawaii
NifTAL Project, Paia, Hawaii; CSIRO Division of Tropical Crops and Pastures,
Brisbane, Australia; USDA Cell Culture and Nitrogen Fixation Laboratory,
Beltsville, Maryland. Of the strains
specifically identified in this report, those with a prefix TAL, IQ, or M are
from NifTAL, and those with a prefix CB are from CSIRO. Most of the strains have been shown to be
effective on at least one of the following legume hosts; Vigna unguiculata,
V. radiata, Arachis hypogea, Stylosanthes guyanensis. Cultures were maintained on yeast mannitol
agar slopes, refrigerated, with 3‑monthly renewal and purity checks
(Vincent 1970).
Culture Media
The
media, all liquid, are described in Table 1.
Acid media were acidified with RU before autoclaving. Aluminum was added after autoclaving as a 5
mM AlK(S04)2 or AlCl3 solution sterilized by
passage through a membrane filter with 0.20 uM openings. Measurements of pH on additional samples
just prior to inoculation showed that neither autoclaving nor addition of Al
changed the pH of the acid solutions. Aluminum and phosphate levels were
designed to avoid precipitation of aluminum phosphate, according to published
solubility criteria and experimental work in similar solutions (Munns
1965a). Samples of uninoculated medium
(c) in experiment A were taken for analysis after centrifugation (Munns 1965b)
and analyzed for P by the colorimetric method of Watanabe and Olsen (1965) and
Al by the 8‑quinolinol method of Frink and Peech (1962). The analysis indicated 95% of the added P
and all of the added Al recoverable in solution in the supernatant. Experiment
A
Six
strains from the cowpea miscellany were selected for growth studies in defined
liquid media at pH 4.6. Three
treatments were imposed (Table 1).
Media were dispensed in duplicate 100 ml volumes into 250 ml Erlenmeyer
flasks, plugged with cotton, covered with a small beaker, and autoclaved for 20
minutes. Bacteria from agar
slopes of similar age were
suspended and serially diluted so that introducing l ml into flasks would give
an initial density of 103 to 104 cells per ml. The diluent contained equivalent
concentrations of MgS04 and CaCl2 at an ionic strength
similar to that of the media. One ml of
the dilution was used to inoculate each replicate treatment. The inoculated cultures were then incubated
on a slowly reciprocating shaker in a constant temperature room at 25°C.
Rhizobial
population density was determined by the agar plate method (Vincent 1970) for
total viable cells, after serial dilution in routine yeast mannitol
medium. Counts were made on duplicate
plates of 30 to 300 colonies per plate after 6 to 10 days' incubation. Colonies
were counted under a low‑power dissection microscope. Where the highest dilution exceeded 300
colonies per plate, a transparent square‑centimeter grid was placed under
the plate and individual squares totalling 10% of the plate area were
counted. Viable counting, though
tedious, was necessitated by the low population densities required to maintain
control of pH and Al (see below).
At
intervals throughout the first 12 days of the trial, all treatments were
sampled for rhizobial density and pH.
In‑sampling for cell density, either 1 ml or 0.1 ml was taken using
a sterile serological pipette. To
saturate the inner wall of the pipette with bacteria, the sample was drawl up
and released eight times before transferring the final sample to diluents or
petri plate for counting. In sampling for pH, a 3 ml volume was removed
aseptically and tested with a combination glass electrode.
Experiment B
Sixty‑five
strains of rhizobia, 52 from the cowpea miscellany and 13 from R. japonicum,
were tested for response to low pH, low P, and high Al in liquid media. Five treatments were applied (Table 1). In addition, the strains of R. japonicum
were also screened in medium (d), adjusted to pH 4.8, and a medium of the same
composition with 25 µM Al at pH 4.8.
All strains were examined twice daily for detectable turbidity, over a
25 day period. A few strains were
selected for detailed sampling for
viable cell count and pH‑change
over an 18 day period.
Media,
inoculation, growth conditions and counting were as in Experiment A, except
that 50 ml volumes in 100 ml flasks were used for the cultures subject to
detailed sampling, and the rest of the units were 5 ml volumes dispensed into
10x135 mm screw‑cap culture tubes. These tubes were incubated in slanted
position atop the shaker. Visually Detectable Turbidity as a Measure of Growth
This
screening trial required a reliable measure of growth less cumbersome and time‑consuming
than viable‑counting.
Nephelometry would be too insensitive, requiring densities of the order
107 per ml or higher (Vincent 1970). It was supposed, however, that if a culture inoculated at only 103
or 104 cells/ml attained visually detectable turbidity, about 107
cells/ml, this would indicate considerable growth, perhaps with no significant
shift from nominal pH and therefore no precipitation of Al, at least during the
earlier stages. It was necessary to
establish two relationships: (i) the correlation of cell density with visual
turbidity, and (ii) the dependence of pH‑change on the increase in cell
density. The first relationship would
yield an average cell density associated with attainment of turbidity. The second would indicate whether growth occurred prior to any change in pH by alkali‑producing strains, or only
after it. Therefore paired counts and
pH, and counts and turbidity observations, were taken n both experiments, A and
B. In studying pH-change with growth,
13 separate pairs of samples were taken from 6 different acid media for 7
strains. For measuring cell density
attained at turbidity, 19 separate samples were taken from 5 different acid media
for 11 strains. The acid media here
included a low‑Ca medium (50 µM Ca) and a high‑Mn medium (200 µM
Mn), both at pH 4.5.
Not all 65 strains were screened at one time; a trial usually
included 15 to 20 strains. To test
consistency of treatment effects in time between trials, strains TAL174N and
TAL209 were included in all treatments in each screening trial. They consistently became turbid at about the
same growth time in a given treatment. Sub‑Experiment
To
verify that the effect of AlK(SO4)2 was due to the Al
moiety, its effect on TAL174N was compared with that of AlCl3. Medium (d) of experiment B, modified by
supplying Fe as FeCl3 (5 µM) instead of FeIIIEDTA,
received no Al or additions of 25 and 50 µM Al as either AlCl3 or
AlK(SO4)2 (fig. 4).
Treatments were in triplicate.
Culture volume was 5 ml.
RESULTS
Relationships off
Turbidity and pH‑Change with Growth
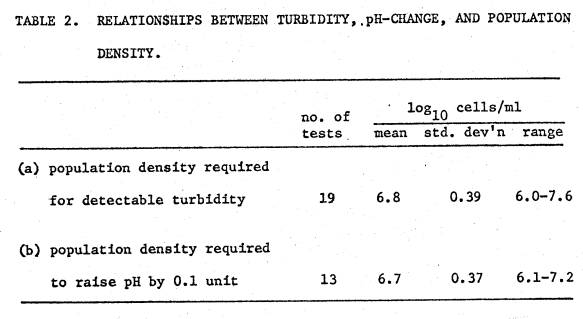
Table
2a shows cell density associated with detectable turbidity in acid media was
consistently ~107 cells/ml.
All cultures in this investigation were inoculated at levels between 103
and 105 cells/ml, most below 104. Therefore, from 2 to 4 orders of magnitude
of growth occurred before the culture became turbid. With the restriction that initial inoculum level has to be <
105 cells/ml, as determined by viable count, attainment of visually
detectable turbidity is a reliable criterion of significant growth.
Table
2b shows that the population required to significantly raise the pH of the
medium was also about 107 cells/ml.
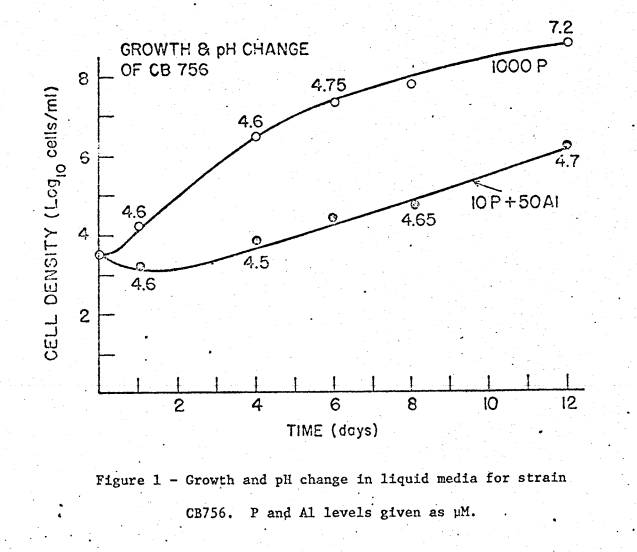
An example of pH change during growth in acid media is given in fig.
1. Though almost all the strains
eventually produced an alkaline reaction, measurements taken throughout the
growth period showed that the rhizobia had to make several fold growth before
changing pH by 0.1 unit. The pH rose
rapidly only when densities >107/ml were reached.
Evidently, strains which made good growth in acid or acid‑Al media could
increase in number by up to 4 orders of magnitude before appreciably changing
the pH and precipitating Al. This
reflects real tolerance to the stress factors.
The rhizobia did not first raise the pH and then grow.
Detailed Growth Studies
Results
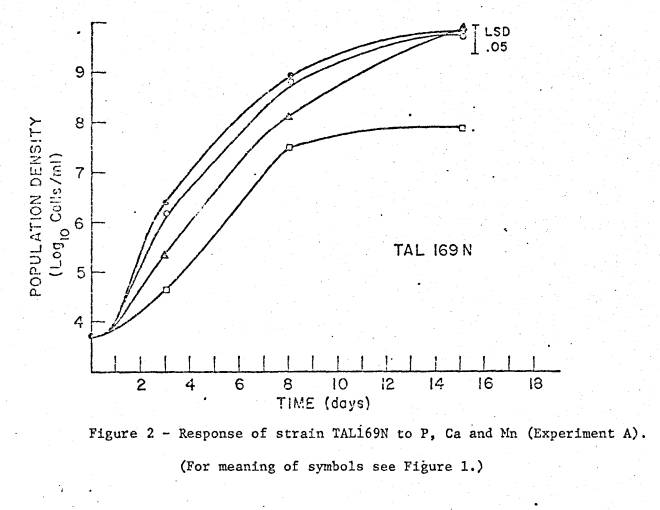
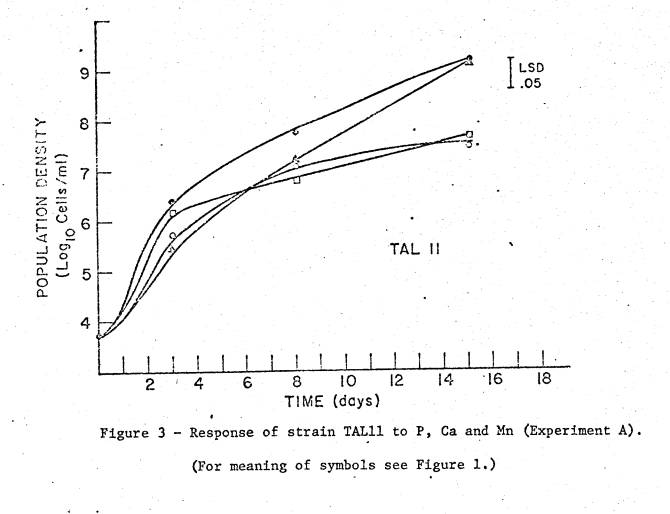
of the detailed growth studies in experiments A and B are shown in figs. 2 and
3. All the strains were in the cowpea
miscellany. Marked variation between
strains in response to both P and Al is
manifested in varying lag times, as well as reduced growth rates whether or not
preceded by a lag.
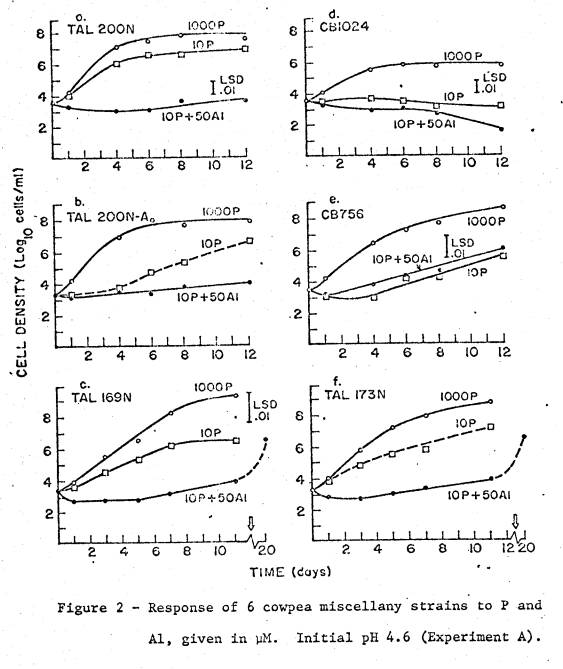
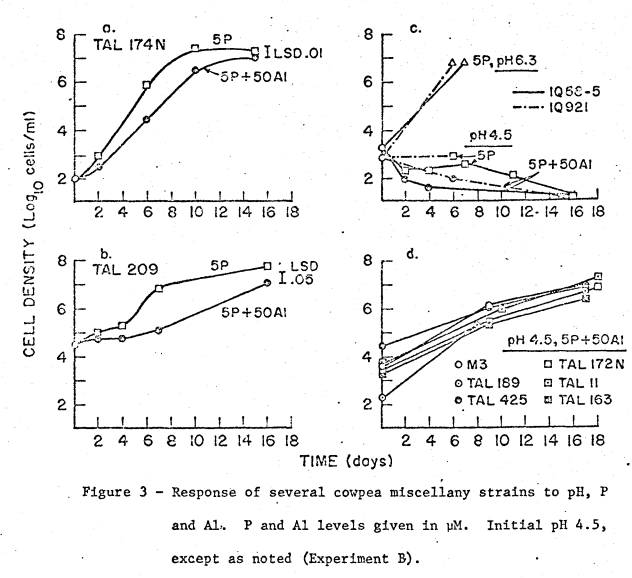
Two
straits, IQ68‑5 and IQ921, were acid sensitive, unable to grow at pH 4.5
(fig. 3c). Strain CB1024 was semi‑sensitive
to acid, unable to make vigorous growth at pH 4.6 even with 1 mM P (fig. ld).
This strain responded slightly to inclusion of vitamins in non‑stress
medium, but not at low pH. In the remaining,
acid‑tolerant strains, the presence of 50 µM Al was clearly the
most severe stress.
Lag
periods in low‑P media were usually less that in Al‑media, but
large variation was evident. Strains
TAL200N (fig. 2b) and TAL173N (fig. 2f) both displayed a 4‑day difference
in lag time between replicates at 10 µM p, so that the dotted line indicating
that growth curve is approximate. In
general, replicates varied in growth much less in nonstressed treatments than
in treatments that imposed P or Al stress.
Generation
times were calculated over the period of exponential growth, usually from day 1
to day 4, following any lag period. For
acid‑tolerant strains, the mean generation time in 1 mM P
was 8.5 + 1.2 hours. In low P
media (5 or 10 µM), generation times ranged from 10 hours for TAL174N (fig. 3a)
to 23 hours for CB756 (fig. 2e). In 50
µM Al the generation times ranged from 13.4 hours for TAL174N (fig. 3a) to over
99 hours for TAL200N (fig. 2a).
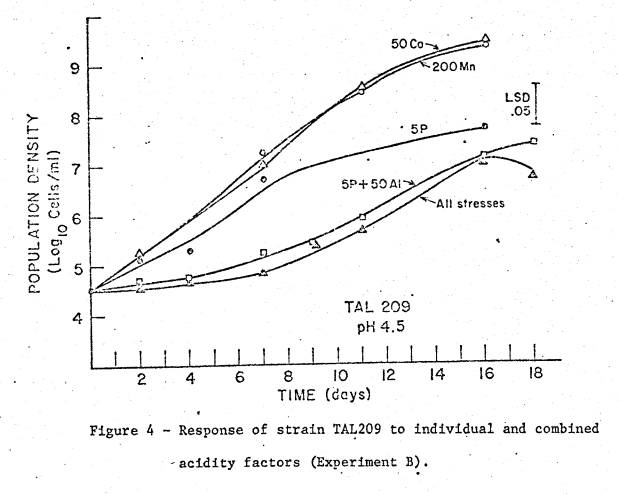
Figure
3d shows the response to 50 µM Al by six strains that had similar high
tolerance, comparable to strains TAL209 (fig. 3b) and CB756 (fig. 2e). In low‑P media, with or without Al,
the maximum cell density attainable usually was between 107 and 10u89
cells/ml, P apparently becoming exhausted at this level.
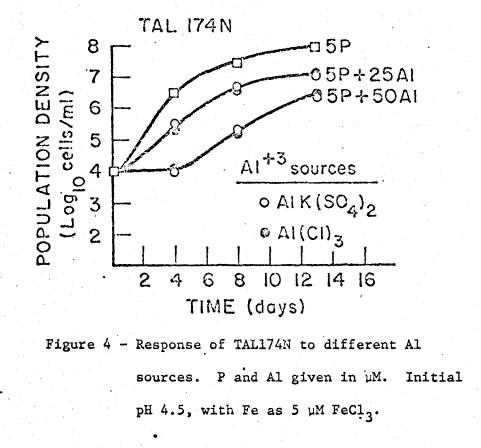
Figure
4 shows the results of adding Al as two different sources on growth of TAL174N. The equal effect of both sources verifies
that the inhibitory effect of the Al compounds was due to Al.
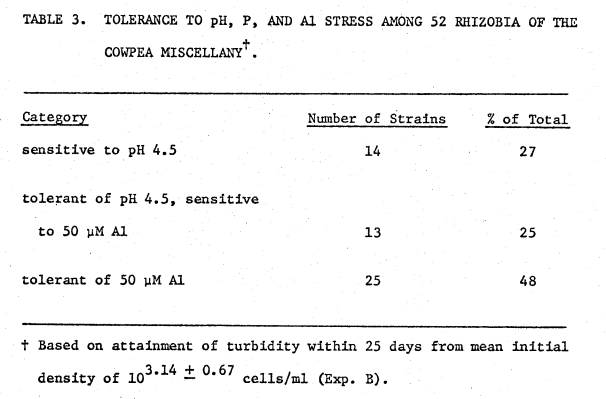
Table
3 lists tolerances to the various factor combinations among strains of the
cowpea miscellany. The data were
combined from detailed sampling trials and the screening trials with growth
assessed by visual turbidity. The data
are based on 25 days' growth from an average inoculum level of 103.14 +
0.67 cells/ml. With 5 µM P, the
average time to reach turbidity was 8.3 days at pH 6.3, 10.2 days at pH 4.5,
and 13.4 days at pH 4.5 + 50 µM Al.
These values are derived only from the strains that grew in the given
medium within 25 days.
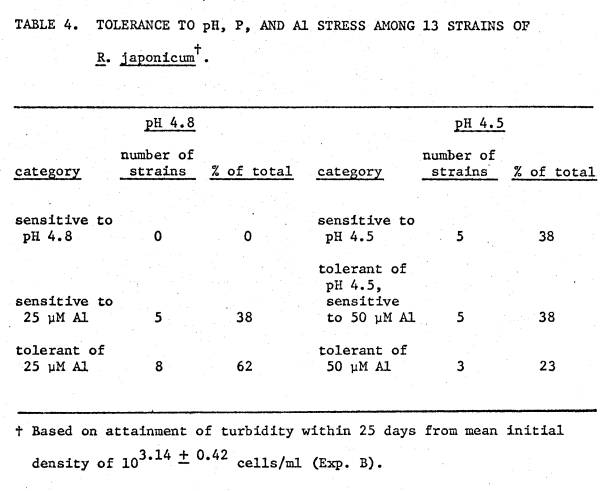
Table
4 shows a similar summary for R. japonicum. The time period was again 25 days, and the
inoculum level was 103.14 + 0.42 cells/ml. At 5 µM P, the average time to achieve
turbidity was 7.0 days at pH 6.3, 11.3 days at pH 4.8, 17.3 days at pH 4.8 + 25
µM Al, and 15 days at pH 4.5 + 50 µM Al.
In yeast and control defined media, the tunes were respectively 5.2 and
7.3 days.
DISCUSSION
The
data show that Al is a potent stress to the growth of free living
rhizobia. Even for tolerant strains, Al
reduced growth rate, and often lengthened lag phase. The data do verify recent evidence that some strains of slow‑growing
rhizobia can survive high concentrations of Al (Rerkasem 1977; Carvalho 1978);
but the large reduction in growth rate shown here could be critical for
colonization of soil and rhizosphere, and for induction of modulation (Munns
1968a; Vincent 1974; Dart 1976).
Aluminum has been clearly shown to inhibit modulation of Stylosanthes
spp. (Carvalho 1978).
The Al concentrations
imposed in these trials were chosen from data of displaced solutions from acid
soils (Pearson 1975; Pearson and Adams 1967).
Also, the 50 µM concentration corresponded to an Al activity of 21.8 µM
calculated from the first‑approximation Debye‑Huckel equation
(Adams 1974), and this activity is well within the range found in acid soil
solutions (Pearson 1975). Thus the 50
µM Al level is a realistic one that rhizobia might encounter in acid soil.
Acidity
itself was a severe stress, preventing growth of about one third of the
rhizobia. Tolerance of acidity did not
necessarily confer tolerance of Al; about half the strains tolerant of pH 4.5
could not tolerate the Al toxicity that would normally be associated with the
acidity in soil. Tolerance to both
acidity and Al was rated at low (5‑10 µM) P, because the high (1000 µM) P
concentration precludes the existence of toxic Al concentration and in any case
is only remotely likely to exist in soil.
The
low P concentration itself inhibited growth of some strains, but with less
severity than acid or Al. According to
data from soil studies (Fox et al. 1978), the 5 µM P
concentration would not be deficient for plant growth. In fact, most soil solutions contain P at
concentrations <1 µM (Reisenauer 1966; Gilman and Bell 1978). Soils, unlike the test media, are buffered
with respect to phosphate. Only in
laboratory media would rhizobia normally encounter the extremely high
concentration of 1000 µM. As Parker et
al. (1977) pointed out, conventional bacteriological media, which are
absurdly rich, may encourage rhizobial dependence on luxury levels of
nutrition. The data here suggest that
the large majority of rhizobia could grow adequately at P levels 100 to 1000‑fold
more dilute than in common media (Vincent 1970; Bergersen 1961). Routine use of media at more dilute nutrient
levels might keep in check any tendency of the bacteria to develop luxury
dependence.
Comparison
of tolerance categories for the two groups of rhizobia suggest that the cowpea rhizobia have more tolerance to Al. However, there were perhaps too few strains on which to judge R.
japonicum. Nonetheless, both groups show similar features. First, within each group there is strain to
strain variation in tolerance; second, acid-tolerance and Al‑tolerance
are separate, as they are for higher plants (Munns 1965b; Andrew et al. 1973; Carvalho 1978);
and third, Al at realistic concentrations appeared to be more commonly a severe
stress than low pH or low P at the levels tested here.
This
study was limited to slow‑growing rhizobia. Some fast‑growing strains might also have useful acid and
Al‑tolerance. Studies from soils acid enough to support moderate
concentrations of soluble Al suggest that R. trifolii might
contain such strains (Munns 1965a; Jones 1966; Jensen 1969).
The
screening procedure of experiment B may prove to be a useful and simple method
for detecting tolerant strains. It
obviates the need for pH control during growth by the simple expedient of using
a small inoculum, so that population density remains too small to raise the pH
and precipitate Al until detectable turbidity is approached. Since Parker
(1971) has shown that pH change in media by rhizobia is a function of organic
composition, an improvement in the screening procedure might be to alter the
media to prevent a large pH change. A
desirable modification also might be reduction of the EDTA concentration to 10
µM. The response of TAL174N to 50 µM Al
was less in the presence of FeIIIEDTA than in the presence of FeCl3
(compare figs 4 and 2a). This may be an
effect of EDTA. Ferric EDTA was used
because if would not interfere with availability of P, whereas FeCl3
could exceed the solubility of Fe(OH)2H2PO4
(Norvell 1972). The stability constant
of FeIIIEDTA so greatly exceeds that of AlEDTA that essentially no
Al complex should form at pH 4.5 (Norvell 1972). However, after making
sufficient growth in the presence of Al, the rhizobia might separate and absorb
enough Fe to allow EDTA to start complexing Al. If so, the use of EDTA would diminish the effects of Al.
The
importance of saprophytic competence in rhizobia has been emphasized by Chatel et
al. (1968) and Parker et al. (1977). Introduced bacteria
often are unable to tolerate biotic or abiotic stresses in a new environment
(e.g., Alexander 1971). Identification
of strains of Rhizobium having superior tolerance to mineral stresses
may be a step towards improving chances of selecting successful inoculants for
acid infertile soils. It now needs to
be shown if rhizobial tolerances to acidity factors in pure culture relate to
their tolerance in soil.
SECTION
II
EFFECTS
OF CALCIUM AND MANGANESE IN COMBINATION
WITH
OTHER SOIL ACIDITY FACTORS ON GROWTH OF RHIZOBIA
INTRODUCTION
The
requirement for Ca as an essential nutrient for rhizobia is quite small, as
determined in liquid media (Loneragan and bowling 1958; Norris 1959; Bergersen
1961; Vincent 1962). Vincent (1962)
showed the Ca requirement to be about 25 µM for normal growth, and found no
effect of pH down to 5.5 on the response to Ca. Loneragan and Dowling (1958) found no response to Ca from 0.1 to
10 mM for R. trifolii at various pH down to 4.5 which stopped
growth. However, Rerkasem (1977)
reports that Ca prevented the effects of moderate acidity for fast growing
rhizobial strains, while cowpea miscellany strains were more tolerant of
acidity and displayed no response to Ca at low pH. Further, in soil at pH 4.5, addition of a neutral Ca salt did not
affect growth or survival of a fast or a slow grower, but did improve the
growth of the fast grower in the rhizosphere.
While
Ca can partially ameliorate the inhibitory effects of Al on non‑symbiotic
legumes (Munns 1965b; Foy et al. 1969; Lund 1970), there is
little information of such an interaction on rhizobia. The one relevant study is that of Rerkasem
(1977) where 1 mM Ca prevented the decline in viability of a fast grower in
solution at pH 4.3, but did not overcome the negative effects of Al
addition. A slow grower that was not
affected by the acidity or Al did not respond to Ca either.
Rhizobium
strains differ in their tolerance to acid soils with Mn toxicity (Dobereiner et
al. 1965; Dobereiner 1966).
Rhizobia can tolerate very high levels of Mn in artificial media
(Dobereiner 1966; Ma Masterson 1968; Holding and Lowe 1971) but there appears
to be no information from actual growth studies concerning effects of high Mn
at low pH.
The
objectives of this research were (i) to examine the effects of low Ca on
rhizobia at low pH, and at low pH in combination with high Mn and Al and low P,
(ii) to examine the effects of high Mn at low pH, and at low pH with high Al
and low Ca and P, (iii) to compare these effects with those of low P and low P
+ high Al from Section I and (iv) to determine any effects of increasing Ca
levels on the response to Al among rhizobia.
MATERIALS
AND METHODS
Rhizobia and Culture Media
Section
I and the Appendix lists sources of rhizobia, and particulars of media
preparation, adjustment of Al and pH, and counting of viable cells. The media, all liquid, are described in
Table 1.
Experiment
A
Three
strains from the cowpea miscellany were selected for growth studies in defined
media at pH 4.6. Four treatments were
imposed (Table 1). Media were dispensed
in triplicate 50 ml volumes in 250
Erlenmeyer flasks, plugged
with cotton, covered with a small beaker, and autoclaved for 20 minutes. Bacteria from agar slopes of similar age
were suspended and serially diluted so that delivering 1 ml to treatments gave
an initial density of about 103 cells/ml. The diluent was basal solution adjust to pH 4.6. Population density was determined as viable
cells.
Experiment B
Forty‑two
strains of rhizobia, 32 from the cowpea miscellany and 10 from R. japonicum
were tested for tolerance to high Mn (200 µM) and low Ca (50 µM) (Table
1). Five of the Al‑tolerant
cowpea miscellany strains and all 10 of R. japonicum were further
tested in a combination medium having the low Ca and high Mn along with low P
(5 µM) and high Al (25 or 50 µM) (Table l).
The treatments were adjusted to pH 4.5 for cowpea miscellany and 4.8 for
R. japonicum strains. In
the combination treatment the Al levels were 50 µM for the cowpea group and 25
µM for R. japonicum. All
strains were examined twice daily for detectable turbidity over a 25 day
period. One strain was sampled for
detailed study over an 18 day period.
Duplicate 5 ml volumes were dispensed in screw cap culture tubes, and
the diluent was basal solution adjusted to the same pH as that of the
given medium. Experiment C
Three
strains from the cowpea miscellany were tested in a factorial combination of 3 Ca and 4 Al levels at pH 4.5
(Table 1). Samples were taken over the 2 1/2 week growth period for viable
counts. Triplicate 5 ml volumes were
dispensed in screw cap culture tubes, and the diluent was basal solution
adjusted to pH 4.5.

RESULTS
Results of the growth
studies in Experiment A are shown in figures 1 to 3. The 10 µM P medium limited final cell density in all 3 strains,
as did 50 µM Ca for TAL11 (fig. 3). High
Mn (200 µM) did not limit final density in any strain, but did reduce growth
rate of TAL11 and TAL169. Sampling
dates were not frequent enough for calculation of growth rates, but relative
differences in rates are apparent in the growth curves.
Tables
2 and 3, and fig. 4 show results of Experiment B. In general, 50 µM Ca or 200 µM Mn had much less effect than 50 µM
Al (Section I), though for some strains their combination in the presence of Al
had effects beyond that of the Al alone.
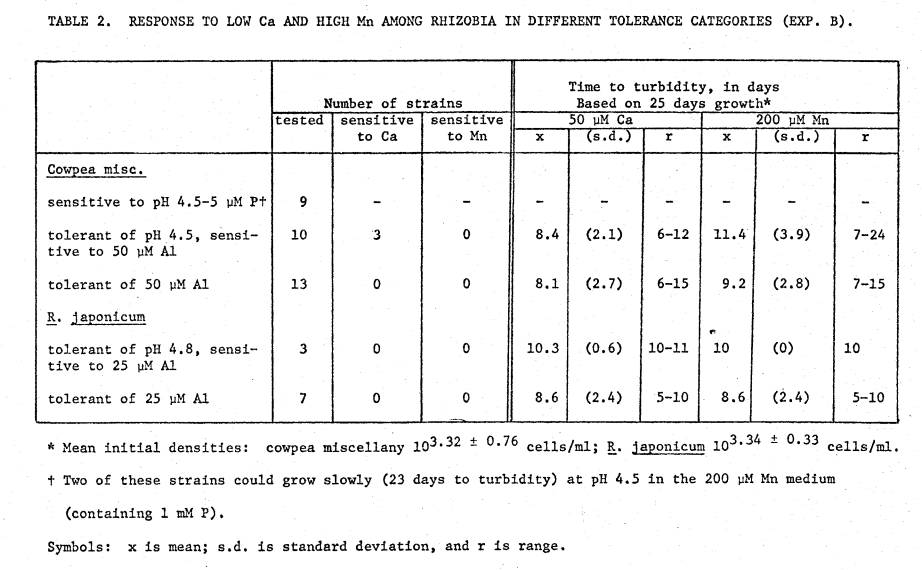
Table
2 lists tolerances to low Ca and high Mn among the 2 groups of rhizobia. For comparative purposes, tolerance was
judged as in Section I, i.e., by the strain's ability to achieve turbidity in
25 days from an initial low level inoculum.
For the cowpea miscellany 2 strains previously shown to be acid‑sensitive
at 5 µM P were able to make turbid growth at pH 4.5 and 200 µM Mn, albeit only
after 23 days. Since this high Mn medium contained 1 mM P, these 2 strains
appear to be more tolerant of acidity with very high P. The remaining strains assessed as acid‑sensitive
with 5 µM P were also sensitive here with high Mn. Of the strains previously recorded as acid tolerant but Al‑sensitive,
none were sensitive to high Mn, while 3 were sensitive to the low Ca. Of the Al‑tolerant strains, none were
sensitive to either the Mn or Ca.
Overall, Ca and Mn had little effect on growth rate in the R. japonicum
strains tested.
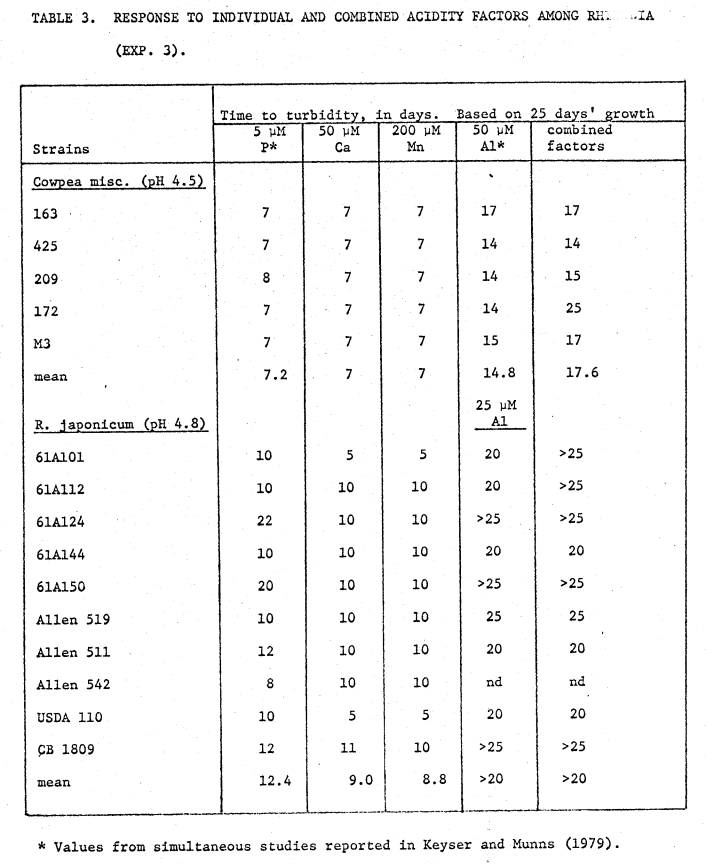
Table
3 lists the time taken to achieve turbid growth in the different stress media,
mainly for comparison with the results of the combined factors treatment. For the 5 cowpea strains low Ca and high Mn
provide little stress, but strains 172 and M3 displayed a significant
inhibition beyond that of the Al alone when the low Ca and high Mn were also
included. This was also noted for 2 R.
japonicum strains.
The
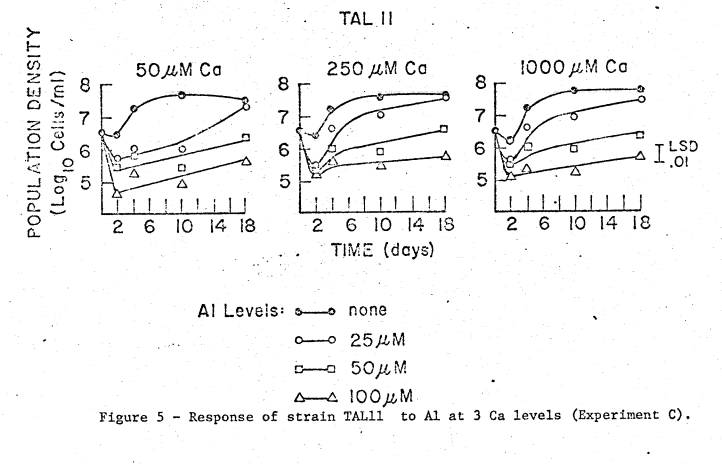
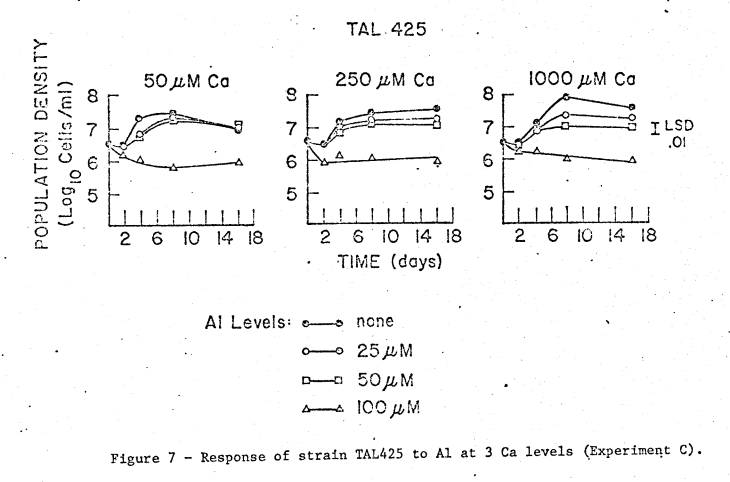
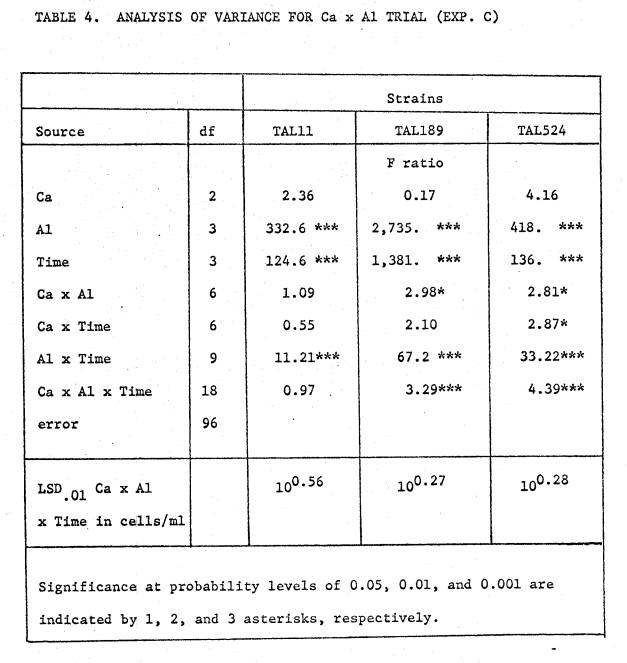
results from the Ca x Al trial are shown in figures 5 through 7, and a
summarized analysis of variance is given in Table 4. Though statistical analysis indicates significant Ca interaction
effects for 2 of the 3 strains, inspection of the growth curves suggests that
Ca offers too little protection against Al to be biologically significant.
The
statistical analysis for TAL11 shows no main or interaction effects of Ca. All levels of added Al caused a significant
early reduction in viable density, with the 25 µM Al treatment thereafter
showing a faster growth rate than the 2 higher levels. The low P level in this trial limits total
cell number and therefore prevents the response to Ca that TAL11 showed in
Experiment A.
For
TAL189, the initial large decrease in viability occurred only at the 2 highest
Al levels; however, this strain was able to recover rather well, though at
lower growth rates. Statistically, the
effects here are also largely due to Al levels and time, but there were smaller
effects of Ca in first and second order interactions. The Al x Ca effect appears to be due to a slight progressive
response to increased Ca levels only at the highest level of Al (100 µM), this
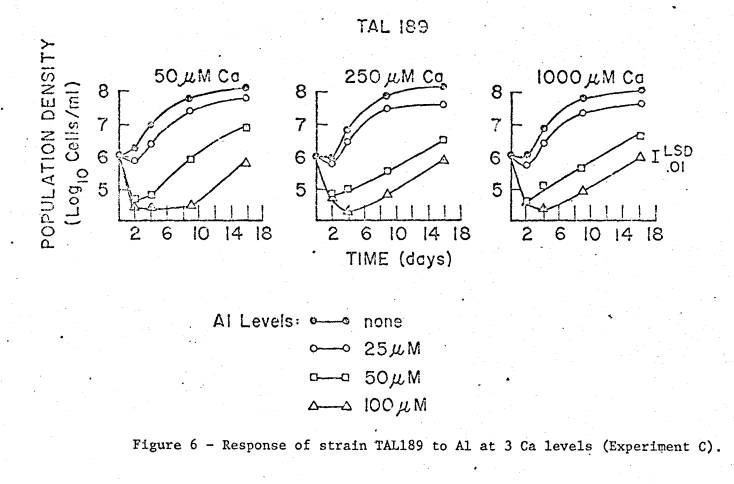
determined from comparing
all Ca‑Al means averaged over time.
From inspection of all individual means, the significant second order
interaction appears to be due to the longer lag period in the lowest Ca and
highest Al level as compared to the 2 higher Ca levels at the same Al
level. However, the 50 µM Al level at
the lowest Ca addition grew slightly faster than at the 2 higher Ca levels, so
that a meaningful trend is not apparent.
For
TAL425, the results are more statistically complicated. The simple features are that even at 100 µM
Al there was comparatively little initial decline in viability, there was good
early growth with up to 50 µM Al, and the Al‑free treatment displayed the
greatest Ca response. From inspection
of the appropriate means, the first and second order Ca interactions appear to
be due to the combination of the increasing response to Ca for the 0 and 25 µM
Al treatments, and the slightly contrasting behavior over the Ca range at 100
µM Al. While this strain displayed the greatest Ca effects on Al response, the
dominating effects of Al level are still clear.
In
the Ca x Al trial, Al activities were calculated using the first approximation
of the Debye‑Huckel equation (Adams 1974). Increasing Ca concentrations
did not seriously lower Al activities through an effect of ionic strength. In the 25 µM Al treatment, the Al activity
ranged from 11.4 µM at the lowest Ca level, to 10.5 at the highest Ca. The corresponding ranges of Al activity were
22.7 to 20.8 for the 50 µM Al media, and 45.0 to 41.4 for the 100 µM Al media.
The
initial declines in counts for strains TAL189 and TAL11 were probably due to death of cells, not to
clumping. Aluminum‑induced
clumping, observed by Rerkasem (1977) at high Al concentration (1 mM), was
restricted to fast growing strain, not slow growers. Further, phase‑contrast microscopy of the suspensions in
the Ca x Al trial indicated that almost all cells were isolated from each
other.
DISCUSSION
At
low pH, 50 µM Ca and 200 µM Mn imposed little if any stress to the majority of
cowpea miscellany and R. japonicum strains. The data confirm earlier work of Vincent
(1962) where Ca deficiency for several strains did not occur above a level of
25 µM at pH 5.5, though in the present study a few acid‑tolerant strains
were detected which did not make turbid growth with only 50 µM Ca. Since these same strains were able to make
turbid growth at low pH with 300 µM Ca (in the high Mn treatment; also in
Section I) it appears that some strains will respond to Ca beyond a level of 50
µM at pH 4.5. A similar response was
found by Rerkasem (1977) for some fast growing rhizobia.
None
of the acid‑tolerant strains were limited in final densities by the high
Ma level, though some did display early depressive effects on growth
rates. Rhizobia have been shown to
tolerate levels up to 16 mM in artificial media (Dobereiner 1966; Masterson
1968; Holding and Lowe 1971).
Comparison
with results of Section I implies that Al is the most severe stress at the
levels tested, and all strains tolerant of Al were also tolerant of low Ca and
high Mn. An additive negative effect of
these combined factors was found for a few of the Al‑tolerant
strains. This may be of significance
since all these factors could occur together in an acid soil (Munns 1977a and
b).
For
growth of legumes, Ca levels from 1 to 5 mM can ameliorate the effects of Al
(Munns 1965b; Foy et a1. 1969; Lund 1970), but for the 3 rhizobia
tested here any beneficial effects of increased Ca up to 1 MM were slight. This agrees with similar observations on
other Al‑tolerant strains (Rerkasem 1977). Also, as Helyar (1978) pointed out, some plant studies claiming
protective effects of high Ca did not take into account large effects of Ca
salts on the activity of Al. Mostly, the data here confirm that Al can be quite
inhibitory to rhizobia, in some strains causing an initial decline in viability
as well as an increased lag period and a reduced growth rate. An early decline in viability has also been
demonstrated in acid soil (Vincent and Waters 1954a). Ability of strains to recover after a large initial decline in
viability in the presence of Al may imply physiological adaptation or selection
of genetically tolerant variants.
Compared
with soil solution analyses from a wide spectrum of soils, 50 µM Ca is very low
(Reisenauer 1966; Gilman and Bell 1978). It is difficult to find data on soil
solution Mn values associated with Mn toxicity, but the 200 µM level tested
here can be inhibitory to several legumes grown non‑symbiotically in
solution culture (Morris and Pierre 1949; Andrew and Hegarty 1969). Further, comparable levels of these acidity
factors are known to adversely affect the nodulation, nodule function, or
growth of legumes, including those that are hosts for the slow growers such as Glycine
spp., Vigna unguiculata, Arachis hypogea, and Stylosanthes spp.
(Armiger et al. 1968; Andrew and Hegarty 1969; Lowther and
Loneragan 1970; Andrew et al. 1972; Munns 1977, 1978; Andrew
1978; Carvalho 1978). Therefore, under
acid conditions the tolerance to low Ca or high Mn among most slow growing
rhizobial strains appears at least equal or be superior to that of the host
plants, while tolerance to Al is less common.
Finally,
if the more important tolerances among strains could be verified in the soil
environment, then the ability to identify these tolerances for a given strain
would be a valuable aid in interpreting effects of such soil acidity factors on
the legume‑Rhizobium symbiosis.
SECTION
III
ADAPTATION TO ALUMINUM BY RHIZOBIA
INTRODUCTION
Adaptation
among microorganisms to various stresses has been reviewed by Stanier (1953)
and Dean and Hinshelwood (1953). From
their discussions it seems justifiable to define microbial adaptation as a
population's gain is ability to function under stress. Further, there appear to be two prominent
types of adaptive mechanisms, though they may not be entirely mutually
exclusive; (1) selective adaptation, being the selection under stress of
genetic variants already existing in a population, or of new mutants, and (2)
physiological adaptation, being the variable, phenotypic adaptation of individual
cells of the same genotype.
Three
important phenomena of acid‑Al stress on rhizobia were observed in
Sections I and II. First, some strains
displayed early death of cells with no recovery. Second, there was most often a lengthened lag period, this
varying between‑strains; and in some cases within strains at small
inocula levels. During the lag phase
some strains which eventually recovered showed an early decline in cell
numbers. Third, there was
strain-dependent regrowth after the lag phase, quite often at reduced growth
rates. All of these observations have
been found with microbes under stress conditions in general (Jackson and
Hinshelwood 1950; Baskets 1952; Doudoroff 1940). Specifically, Foy and Gerloff
(1972) reported slow growth at high Al levels for a tolerant Chlorella
species. In a study of NaCl stress to
rhizobia, Steinborn and Roughley (1975) attributed the longer time for strains
to grow at high concentrations to the time necessary for adaptation. The number of cells of a R. meliloti
strain able to grow on 3% NaCl agar was only one‑tenth to one‑twentieth
of the population.
As
a preliminary investigation into the nature of such adaptation an experiment
was set up. Several strains of rhizobia
were tested for growth from widely varying inoculum levels in Al‑containing
medium. This approach should demonstrate whether or not growth is due to the
selective adaptation of new mutants.
MATERIALS
AND METHODS
Ten
straits of Rhizobium, 9 of the cowpea miscellany, and 1 R. japonicum
were tested in pH 4.5‑5.0 µM Al medium, the same composition as in
treatment (d), experiment B of Section I.
For each strain 4 levels of inoculum were introduced by 100‑fold
serial dilutions of a high density suspension.
All strains were tested in duplicate at each inoculum level. Strains TAL174N, TAL98A, and TAL209 were
tested in 20 ml volumes in 50 ml tubes.
Viable counts were taken initially on all inocula and at day 17. The diluent composition was that of the
medium less the Al. Details regarding
medium preparation, growth conditions and counting are described in Experiment
A of Section I.
RESULTS
AND DISCUSSION
The
results are shown in Table 1. Three
types of intrastrain response emerge.
Strains TAL174N, TAL209, M3, TAL169N and CB756 were
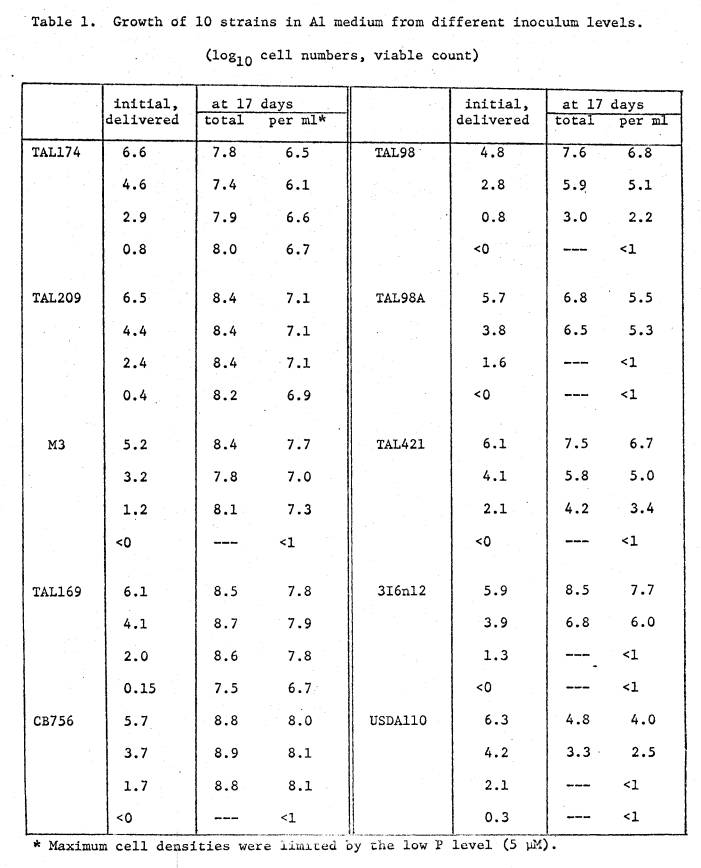
able to attain high levels
from only a few initial cells in the Al medium. Strains TAL98, TAL98A, TAL421 and n12 had a slower growth rate
than the first group, and some did not grow from very low initial levels. R. japonicum US110 showed a
reduction in cell numbers at all inoculum levels.
All
strains in the first group were assessed as Al‑tolerant in Section I,
judged from turbid growth and viable counts from inocula at 103
cells/ml. A simple interpretation is
that these strains contain a large population of cells with the ability to
adapt rapidly in Al‑stressed medium.
Their response at very low inocula levels indicates little if any death
of cells during an adaptive period, or alternatively, few cells genetically
incapable of adapting.
Contrasting
this is the behavior of R. japonicum US110, which was judged
sensitive to pH 4.5 (Section I). It
shows death of cells at all levels of addition, being unable to adapt to the
acid‑Al medium within 17 days.
Of
the remaining strains, TAL421 and n12 were rated Al‑tolerant (Section
I). TAL421 has a very slow growth rate
in Al for an adapted strain. Strain nl2
also has a slow growth rate, and no growth from the addition of only 20 cells
(101.3). This may be due to
death of cells during an adaptation period or alternatively, few cells genetically capable of
adapting. TAL98A received separately
from 98, subcultured from the same parental strain. While both show slow growth rates as compared to the most
tolerant group, TAL98 made growth from fewer cells than 98A. This difference is probably of no
significance. Even from the same
dilution, Jackson and Hinshelwood (1950) found variation in a strain of
bacteria adapting to ammonium sulphate.
From
the data here it seems clear that the adaptation to Al stress is not due to the
occurrence of de novo mutants. Stanier et
al. (1976) and Brock (1974) put the frequency of new mutants at 10‑8
to 10-9, and the probability of this accounting for the growth of
even the less tolerant strains is very small indeed. The argument for selective adaptation of existing genetic
variants is inconclusive from this data.
The performance of the 5 most tolerant strains suggest they have a
uniform population in respect to adaptation abilities. While some of the other
strains did not make growth from very low levels, it can be argued that this
was due to identical cells which did not survive an adaptation period (Jackson
and Hinshelwood 1950), compatible with the mechanism of physiological
adaptation.
This
study concludes that the mechanism of Al‑adaptation is not one of
spontaneous mutation. It shows that
large differences exist between strains in ability to adapt. Also, there appear to be degrees of
adaptiveness, expressed in relative rates of growth from a given inoculum
level, as well as ability to grow from very few initial cells. To elucidate the adaptive mechanism
responsible for tolerance, further studies can now focus on discriminating
between the existence of genetic variants already present or that of phenotypic
adaptation of similar cells. This would
require determining the stability of adapted cells, using such techniques as
replica plating and ability of cells to be trained for adaptation and to retain
the adaptation in the absence of the inducing stress.
SECTION
IV
RELATIONSHIP
BETWEEN RHIZOBIAL TOLERANCES IN PURE
CULTURE
AND SYMBIOTIC PERFORMANCE IN ACID SOIL
INTRODUCTION
Differential
strain tolerance to soil acidity has been demonstrated for different rhizobia
species (Munns 1965a; Lie 1971; Rerkasem 1977; Munns et al.
1978). Lie (1971) reported that a R.
leguminosarum strain that gave the best symbiotic performance at pH 4.6
was a comparatively mediocre strain at neutral pH. This observation was confirmed by Munns et al.
(1978) on several strains of the cowpea miscellany, where effect of acidity on
a strain's performance bore no relationship to its effectiveness at higher pH.
The
detrimental effects of Al on symbiotic legumes are attributed to reduced
nodulation and plant growth (Sartain and Kamprath 1975; Carvalho 1978). For Stylosanthes species, Carvalho
(1978) demonstrated that the nitrogen fixation activity of nodules formed in
the absence of Al was not reduced upon exposure to levels up to 100 µM, but
nodule formation was. Increased
nodulation of soybeans was partly attributed to reduced Al saturation in soil
from liming, while it was also highly correlated with root Ca level (Sartain
and Kamprath 1975). Differential
symbiotic performance of rhizobia under conditions of Al stress have not been
demonstrated.
This
section describes the symbiotic performance in soil of 25 strains of rhizobia
on 3 varieties of Vigna unguiculata (cowpea). Comparative effectiveness of a strain at low
pH with its effectiveness at a more neutral pH (symbiotic acid tolerance) was
then compared with the strains' unknown tolerances to acidity and Al in pure
culture, as determined in Sections I and II.
MATERIALS
AND METHODS
Two
greenhouse pot trials were conducted to test the symbiotic acid tolerance of
several rhizobial strains. Three
cultivars of the acid tolerant legume V. unguiculata were grown
on two soils; one variety on the first soil, and 2 other varieties on the
second soil. The treatments for each soil were a low pH of 4.6 and a more
neutral pH of 6.0‑6.2. Previous
experience with the two soils showed them to be very nitrogen deficient and
naturally acid.
Seed
of Vigna unguiculata cv. Blackeye 5 (BE5) was obtained from the
California Crop Improvement Association, Agronomy Department, University of
California, Davis. Seed of varieties
TVu 1190 and TVu 4557 were supplied by the International Institute of Tropical
Agriculture, Ibadan, Nigeria, by way of the University of Hawaii NifTAL
Project, Paia, Maui. The day of
planting, seeds were surface sterilized by submergence in 30% H202
for 5 minutes, and thoroughly rinsed with distilled water.
Twenty‑five
strains of rhizobia were tested, and are listed in the Appendix. Thirteen strains were used as inocula for a
trial with BE5, and 23 strains with the TVu varieties, with only 2 strains
tested on BE5 not also tested on the other 2 varieties. All of the strains were tested for tolerance
to acidity and Al in pure culture in Sections I and II. For inoculation, cultures from agar slants
were suspended in full nutrient broth (treatment b, Sec. B, Sec. I), diluent,
and delivered to seed in pots immediately before it was covered with soil. Viable counts of inoculum showed that 104.9
+ 0.25 cells per seed was applied in the BE5 trial, except for
strains TAL163 and TAL98 which, not by design, received 106.4 and 106.2
cells per seed, respectively. In the
trial with the TVu varieties 105.9 ± 0.5 cells/seed was
applied. In each trial, a given strain
was applied at the level for all treatments with that strain.
The
soil used in the trial with the BE5 variety was Goldridge loamy fine sand
(Typical Hapludult, fine loamy, mixed, mesic), B‑horizon material
collected from Sebastopol, California.
It had a saturation paste pH of 4.6, and levels of available cations as
listed in Table 1. Mineralogical
analysis of the clay fraction showed it contained predominately kaolinite. Two pH treatments were imposed; the
unadjusted pH of 4.6 and a pH of 6.0 from the addition of CaCO3
Limed treatments received 0.9 grams lime per kg soil. Basal nutrients applied to all treatments were 10 mMoles KH2P04,
2 mMoles K2SO4, 10 mg Zn and 0.1 mg Mo per kg soil. Nutrients and lime were added to 1.9 kg soil
in plastic pots, mixed, watered, and incubated for a week before planting. The final pH of each treatment was checked
the day before planting. Combined
nitrogen treatments received 12 mMoles NH4N03 per pot, in
split applications. A zero control,
with no inoculum or nitrogen, was included, and all treatments were
replicated. Six seeds were planted per
pot, and thinned upon emergence to 3 uniform seedlings.
The
soil used in the trial with the TVu varieties was Josephine silt loam (Typic
Haploxerult, fine loamy, mixed, mesic), B‑horizon material collected near
Georgetown, California. Mineralogical
analysis of the clay fraction showed it contained predominately kaolinite and
oxides. The saturation paste pH of the
soil was 5.4, and available cations are listed in Table 1. Two soil pH treatments were imposed; (i) pH 4.6
by addition of 23.1 me Al as Al2(SO4)3 per kg
soil, and (ii) pH 6.2, by addition of 10.6 me C03 as CaCO3
per kg soil. Basal nutrients applied to all pots were the same as for the
Goldridge soil, except that 12 mMoles KH2PO4 per kg soil
was added, and each pot also received 1.9 kg soil. Nutrients and lime were mixed, watered and incubated for 10 days
with a final pH check before planting.
Combined nitrogen treatments received a total of 16 mMoles NH4N03
in split applications. A zero control
was also included. Nine strains, the +N
and zero controls were tested in triplicate, and the remaining 14 strains in
duplicate. Four seeds of each variety
were planted in the same pot, and thinned upon emergence to two plants per
variety.
Greenhouse
soil temperatures varied between 29°C (day) and 21°C (night). Pots were watered with distilled water, and
maintained between 75 to 100% of the determined field capacity for each soil.
Observations were taken daily on plant color and relative size of foliage. At 45 days in the trial with BE5, and at 42
days in the trial with the TVu varieties, plants were washed out of the soil,
bagged in plastic and refrigerated until examined for nodule number and nodule
fresh weight. Shoots were separated and
dried at 75°C for weighing.
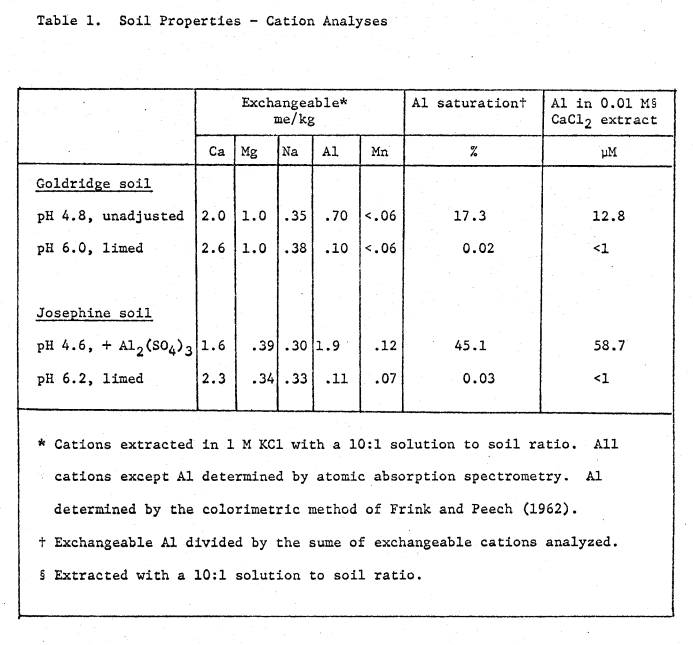
Analysis
of variance was performed on plant yield and nodule number, and for the TVu
varieties also on nodule fresh weight.
To equalize variances when necessary, the transformations 1+log10
yield or log10 nodule number were made for analysis. Plant yield analyses were used to test
associations between rhizobial tolerances in defined acid media with symbiotic
acid tolerance, as defined in the next section.
RESULTS
BE5 variety‑Goldridge
soil
Plants
became yellow, and with effective nodulation became green at the 2nd trifoliate
leaf stage (about 2 weeks) in the limed treatments. The earliest greening of plants at low pH was 3 days behind that
with lime, and some of the more sensitive strains were 2 weeks later.
Figure
1 summarizes the plant yield and nodule number data for the strain in both pH
treatments. Figure 2 compares the
strain dependent yield at pH 6.0 with that at 4.6.
Plant
yield analyses from both treatments were compared using Duncan's new multiple
range test (Steel and Torrie 1960). At
pH 6.0, strains TAL11 and 207 did not yield significantly better than the uninoculated
zero control, and were considered ineffective on this variety. Of the remaining strains, TAL189, 173, 174,
303, 163 and 172 were considered highly effective, not significantly (5% level)
different from the combined N treatment.
Strains CB1024, TAL98, 169, 170 and 171 were moderately effective at
high pH, being significantly better than the zero control, but significantly
poorer than the
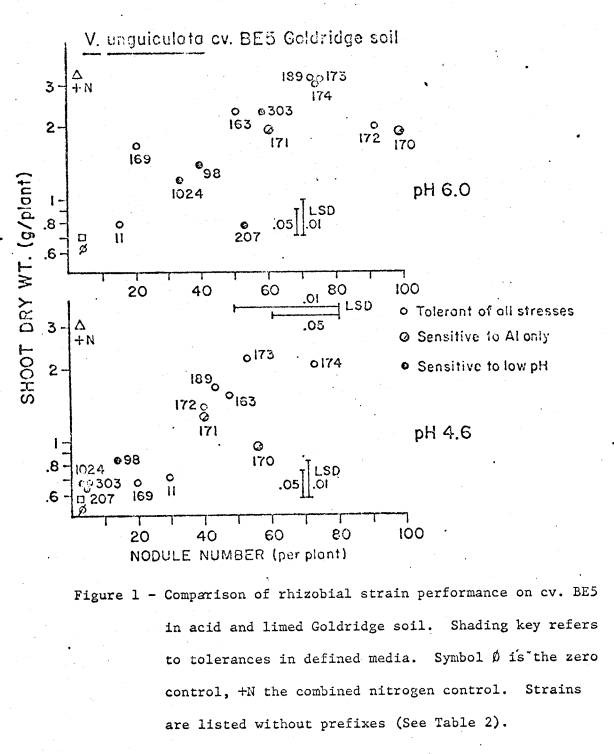
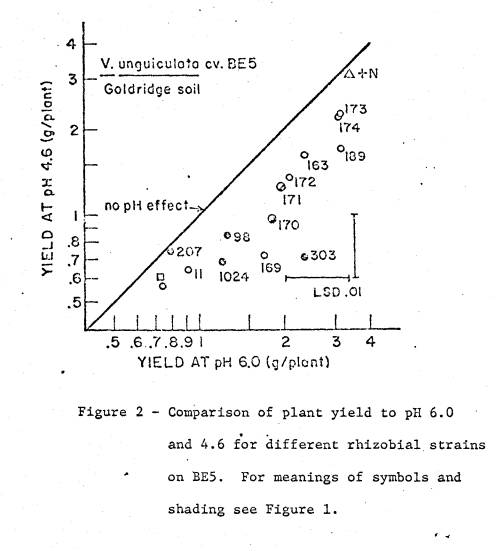
combined N and the highly
effective strains. For effective
strains, there is a good relationship between nodule number and plant yield.
The highly effective group contained one strain which was acid sensitive in
media (TAL303), but obviously had no problem in being able to adequately
survive a soil pH of 6.0. The
moderately effective group contained strains in all 3 media tolerance
categories.
At
pH 4.6, most strains displayed reduced effectiveness and nodule number. Here, only strains TAL173 and 174 remained
highly effective. Moderately effective
at low pH were TAL189, 163, 171, and 172, while TAL169, 303, 98, 170, and
CB1024 were affected to the extent of being completely ineffective.
The
most notable decline in yield due to acidity is found with TAL303, which was
highly effective at pH 6.0 and ineffective at 4.6. The large reduction in
modulation with this strain suggests it survived poorly in the acid soil, which
agrees with its tolerance rating in media.
However, even the very tolerant TAL169 which had few nodules at pH 6.0
behaved similarly to TAL303 at low pH.
More importantly, all the moderately and highly effective strains at low
pH were acid tolerant in media, and all but one (TAL171) were tolerant of Al.
At
low pH, the relationship between yield and nodule abundance appears to be a
closer one than at high pH. This may be
due to a strain's ability to multiply at low pH to sufficient levels for
adequate modulation, though it might also reflect strain‑dependent
limitation in other steps involved in modulation. With the important
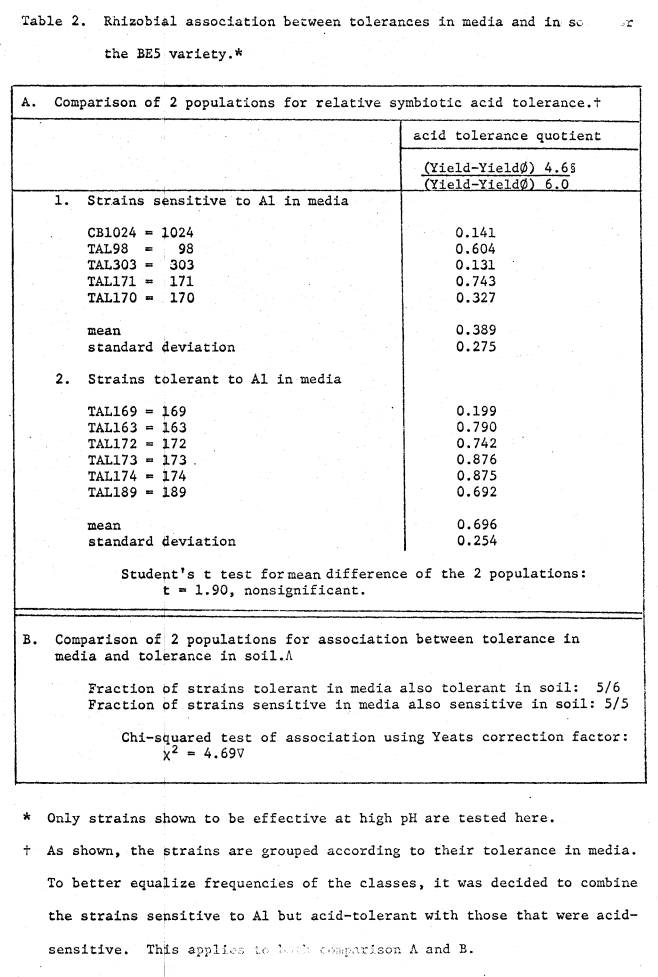

exception of TAL303, there
was also a trend for the strains which performed best at high pH to do likewise
at low pH.
The
nodule fresh weight data related very closely with that of the nodule number
data for all treatments. There was very
little compensation in size for reduced abundance. Therefore, nodule weight data are not presented as they would add
little information.
Table
2 shows the results of comparing 2 rhizobia populations, based on their
tolerances in media, for their symbiotic acid tolerance expressed as an acid
tolerance quotient, and for the association of tolerance in media and in soil
based on counts of observed and expected frequencies. The t test for differences between the two populations for a mean
acid tolerance quotient was not significant, while the X2 test
assuming non‑association between tolerance in media with that in soil was
significant at the 5% level, indicating the association of the 2 properties.
Figure
2 shows in a different way the extreme acid sensitivity of TAL303, indicated by
its vertical displacement from the no pH‑effect diagonal. For all strains it shows their yield
performance at both pH 6.0 and 4.6.
Plate 1 shows the marked N response of BE5 on this soil, and Plates 1 to
5 show the effects of acidity on top growth and modulation for strains TAL173N,
TAL174N, TAL98 and TAL303.
TVu 1190 variety ‑
Josephine soil
Effective
modulation became apparent from the green color of previously yellow plants at
the second trifoliate leaf stage in the lime treatment. As in the BE5 variety trial, there were
varying delays among the strains in this color change at low pH.





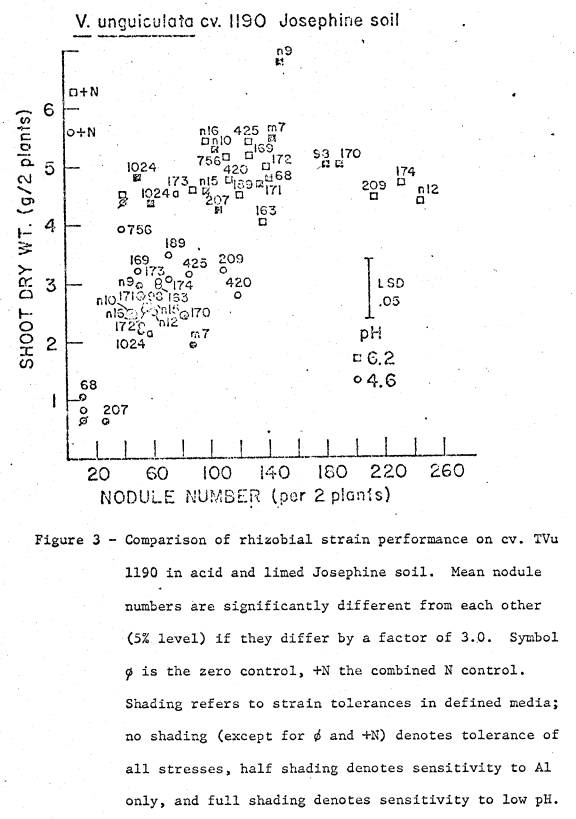
Figure
3 shows the yield and nodule abundance due to each strain in both pH
treatments. Three main features of this
data are apparent. Firstly, there is the 2 pH groupings of the strains for both
yield and nodule abundance, both being larger at pH 6.2. Secondly, the yield from a given strain at
low pH had no useful relationship to its yield at high pH. Thirdly, at pH 4.6, the highest yielding
(greater than 3 g/2 plants) strains were all tolerant of Al in media; CB756,
TAL189, 209, 425, 169, 173 and 174. At
this pH the soil has a high level of Al and these media‑tolerant strains
proved to be tolerant symbiotically.
That this occurred over a fairly large nodule abundance range implies
that high tolerance involves other factors besides just good survival or
ineffectiveness as indicated by nodule number.
Other Al‑tolerant strains, such as n12 and TAL172 had a more
reduced yield at a nodule abundance equal to that of CB756 and TAL169. Also,
the acid‑sensitive strains CB1024 and CB1024a yielded only 50% as much at
pH 4.6 as at 6.2 while having the same nodule number. But as seen in fig. 4, they were much smaller nodules.
Figure
3 also shows that this soil contained indigenous rhizobia which were effective
at pH 6.2. Analysis of yield data with
use of Duncan's multiple range test showed that only one applied strain, n9,
was significantly better than the zero control at high pH. However, at low pH, 20 of the 23 strains
were significantly better, so that the indigenous rhizobia were sensitive to
the soil acidity complex.
Figure
4 shows the relationship between yield and nodule mass. Similar trends are seen
in the data as with nodule number; a general yield response with increasing nodule
mass per plant, the 2 clusters
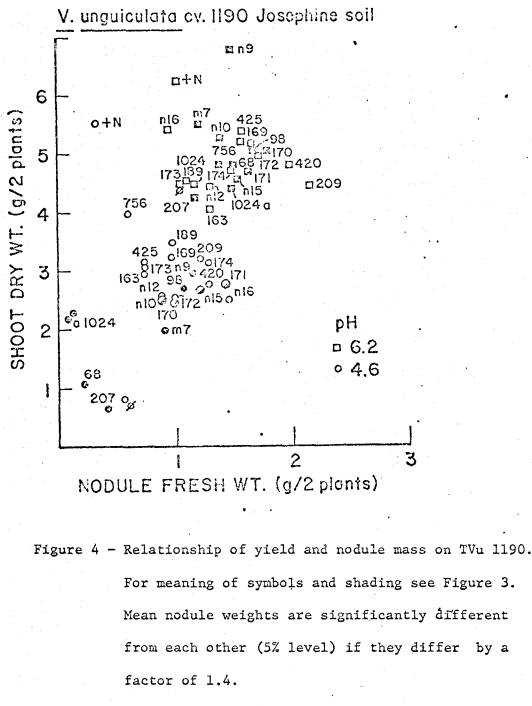
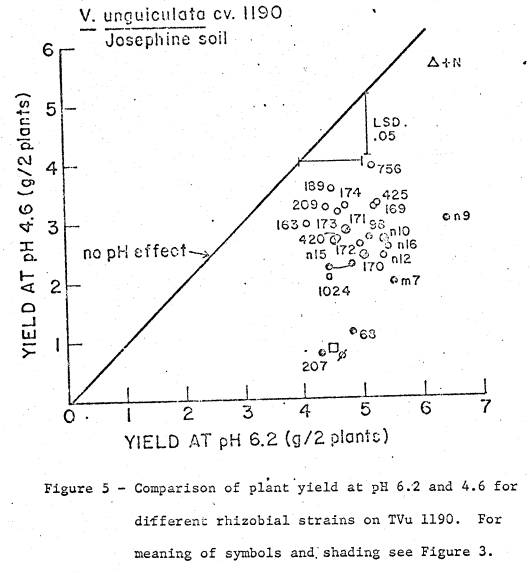
of treatment pH with a
general reduction in mass at low pH, and the unrelatedness of nodule mass at
high pH with that at low pH for a given strain. While nodule abundance and mass generally decreased together at
low pH, the average nodule size tended to increase slightly for the majority of
strains, but exceptions are seen as for the CB1024 strains. Also, the best yielding strain at low pH,
CB756, had relatively low nodule numbers and mass indicating a very high
specific N‑fixation effectiveness for these conditions.
Figure
5 compares the yield of strains at both pH treatments. Distance to the right
relates to effectiveness at high pH, while vertical distance from the diagonal
indicates relative acid tolerance for a strain. It emphasizes again that the best yielding strains in the stress
condition in soil are those which were identified as the most tolerant of
stresses in laboratory media. Strain n9
was classed as acid‑sensitive in media, and while it was better than all
strains at pH 6.2, it showed greater than 50% yield reduction at 4.6. Still, it managed to do comparatively well
at low pH, especially among the acid‑sensitive strains.
Table
3 lists the results of the statistical tests of association as applied in the
BE5 trial. The t test for mean
difference of the 2 populations for net acid yield is highly significant. The chi‑squared test of association of
tolerance in media with that in soil was not significant, the value occurring
at about the 6.5% probability level. It is obvious though that the majority of
strains were associated for these tolerances.
TVu 4557 variety ‑
Josephine soil
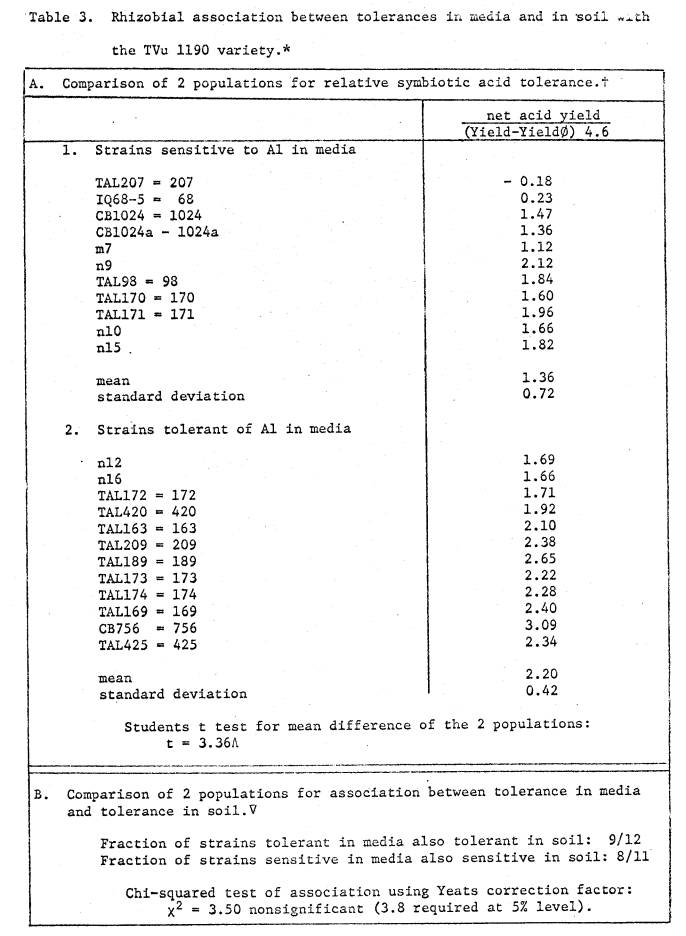


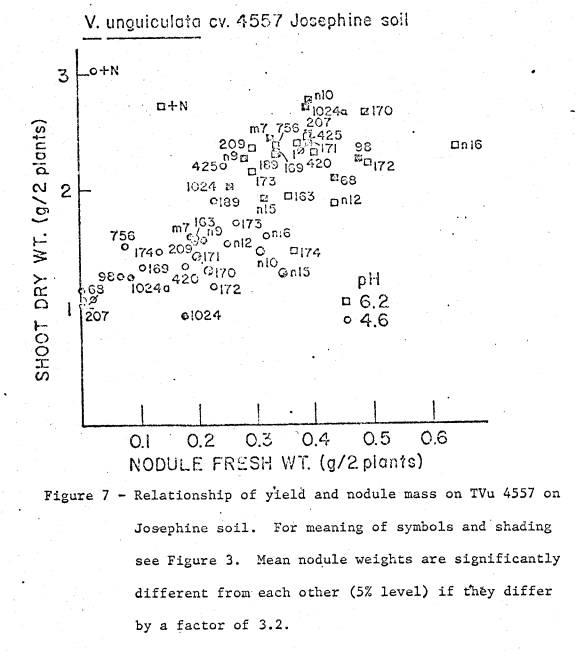
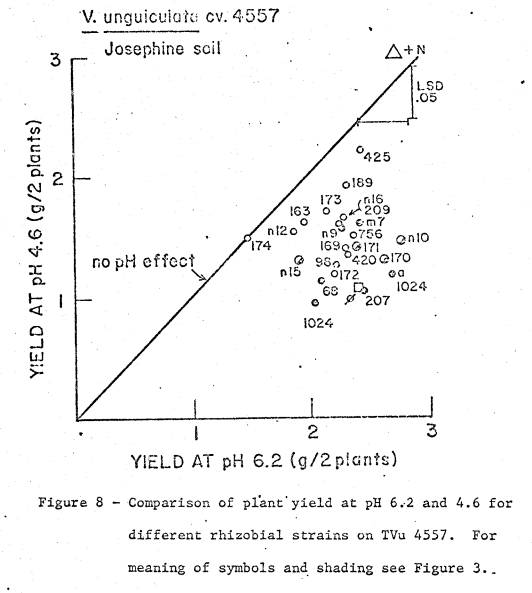


The
time sequence of modulation in both treatments was much the same as for the previous
2 varieties.
Figure
6 summarizes the yield response from the strains as related to nodule number,
and in Fig. 7 as related to nodule mass.
The trends are much the same as for the 1190 variety; a general yield
response with increasing nodule mass and number, these both being reduced by
acidity; the greater sensitivity to acidity of the symbioses as compared with
the nonsymbiotic condition (+N); the unrelatedness of yield at pH 6.2 to that
at 4.6 for a given strain; and the majority of the best yielding strains at low
pH were previously determined to be most tolerant of soil acidity factors.
The
indigenous rhizobia in the Josephine soil were also effective on this variety
at pH 6.2. Analysis of the yield data
using Duncan's multiple range test showed that at high pH no strain was
significantly better (5% level) than this zero control, and 3 strains were
significantly poorer, being then considered ineffective on this variety ‑n15,
n12, and TAL174. At low pH, 10 of the
strains were significantly better than the zero control, though only 2 gave
absolute lower yields than the zero control.
The remaining strains were not significantly different from the control.
Figure
8 shows the comparative yields at pH 6.2 and 4.6 for each strain. Ignoring the ineffective strains (n12, n15,
and TAL174), those with the least relative yield reduction due to effects of
soil acidity are those determined as tolerant of acidity and aluminum. Again,
the agreement between tolerances in media and soil is by no means complete; strains
M7 and n9, which were acid sensitive in media, yielded better at low pH than
such acid‑Al tolerant strains as CB756, TAL169, 172 and 420, while all
had about the same effectiveness at pH 6.2.
Table
4 lists the results of association tests between tolerance in media and
soil. As was found with variety 1190,
the t test shows a significant difference between the 2 populations for net
yield of plants at low pH, while the X2 test of association of
tolerances is not significant.
DISCUSSION
The
data presented here show that there is large strain differences among rhizobia
of the cowpea miscellany for symbiotic acid tolerance on different varieties of
V. unguiculata. Soil
analytical data indicate that the main stress in the acid Goldridge soil is
that of pH per se, while in the acidified Josephine it is the high level of
exchangeable and soluble Al.
Nonetheless, strains which gave the best yield on varieties on either
soil at low pH had the common property of being the most tolerant of acidity
factors as tested in pure culture, especially to Al.
On
studies of symbioses of V. radiata (mung bean) in the Goldridge
soil, Munns et al. (1978) confirmed that it was not necessary to
sacrifice effectiveness to get acid tolerance.
This was also found in the present study on this same soil with the BE5
variety of cowpea.
On
all 3 varieties, the effects of the soil acidity complex were to generally
reduce yield by reducing nodule abundance and mass on the N-deficient
soils. The more severe effects of low
pH on the symbiotic growth of the TVu varieties than with BE5 is attributed to
the high Al level in the Josephine soil.
No nitrogen analysis of plant material was done, as it was clear that
the growth response of the initially yellow plants on these soils was due to the
availability of N through rhizobial association. Also, Munns et al. (1978) have already shown that
the yield of symbiotic V. radiata in the Goldridge soil
correlated well with %N in the tops.
Therefore, the inhibitory effects of soil acidity seen here are
attributable directly to effects on the overall performance of the Rhizobium.
The
drastic effects of pH and Al on nodulation seen here are in agreement with
previous studies (Sartain and Kamprath 2975; Carvalho 1978; Munns et al.
1978). Also, from this present study
there is good evidence that nodulation is reduced because growth of some
strains of rhizobia is impeded by acidity and/or Al.
The
best agreement between strain tolerance in media and in soil is seen with the
highest and lowest yielding strains at low pH. Tolerance in media was for
survival and growth alone. For
symbiotic performance in a stressed soil, other events of modulation or nodule
function may be limited by acidity or Al for a giver strain, a limitation not
related to the ability to make growth in their presence. This may be why some effective (pH 6) and
tolerant (in laboratory media) strains perform poorly in acid soil, despite
adequate modulation. Also, some strains
classed as acid and Al sensitive may simply survive better in stressed soil
than pure culture studies would indicate.
Tests of strain survival alone in pH‑Al stressed soil could clear
up some of these points.
Spain
(1975) has discussed the forage potential of acid, allic soils in South
America. He notes that there are
several tolerant legumes that have promise for good growth on such soils,
provided they are effectively nodulated.
If indigenous, effective rhizobia are not present in these soils, any
inoculum for these plants would have to have the property of Al-tolerance for
adequate saprophytic competence of the bacteria and subsequent host growth on
such soils.
This
is the first reported investigation of the relationship of rhizobial tolerances
to acidity factors in media with that symbiotically in acid soil. Similar work is underway in Colombia (J.
Halliday, personal communication) and it does not seem unlikely that with
experience better relationships will be found.
Even at this early stage of development of such screening of strains for
defined tolerances, it appears to be a promising technique for use in selection
of inocula for acid, unlimed soils. The
value of screening strains for acidity related tolerances in defined laboratory
media is seen in that if a decision was made to eliminate Al-sensitive strains for
use in acid soil trials, then 60 to 70% of the poorest strains would have been
retained for further testing in soil, none of the best yielding strains would
have been eliminated, and this is the right kind of error to have for a pre‑screening
procedure. Obviously, the most critical
test of the validity of such pre‑screening would be its relation with
strain performance in field trials.

BIBLIOGRAPHY
Adams,
F. 1974. Soil solution. IN: E. W. Carson (ed.) The plant root and
its environment. Univ. Press of
Virginia, Charlottesville. p. 441‑481.
Albrecht,
W. A. and F. L. Davis. 1929. Physiological importance of calcium in
legume inoculation. Botanical Gazette
88:310‑321.
Alexander, M.
1971. Microbial ecology.
John Wiley & Sons. New York.
Anderson
A. J. and D. V. Moye. 1952. Lime and molybdenum in clover development on
acid soils. Aust. J. Agric. Res. 3:95‑110.
Andrew,
C. S. 1976a. Effect of Ca, pH and N on the growth and chemical composition of
some tropical and temperate pasture legumes. I. Nodulation and Growth. Aust. J.
Agric. Res. 27:611‑623.
Andrew,
C. S. 1976b. Screening tropical legumes for manganese tolerance. IN: M.
J. Wright (ed.) Plant adaptation to mineral stress in problem soils. USDA
Beltsville, Maryland.
Andrew,
C. S. 1978. Mineral characterization of tropical forage legumes. IN: C.
S. Andrew and E. J. Kamprath (eds.) Mineral nutrition of legumes in tropical
and subtropical soils. CSIRO, Melbourne, Australia. (In press).
Andrew,
C. S. and M. P. Hegarty. 1969. Comparative response to Manganese excess of 8
tropical and 4 temperate pasture legume species. Aust. J. Agric. Res. 20:687‑696.
Andrew,
C. S., A. D. Johnson and R. L. Sandland. 1973. Effects of aluminum on the
growth and chemical composition of some tropical and temperate pasture legumes.
Aust. J. Agric. Res. 24:325‑339.
Andrew,
C. S. and R. K. Jones. 1978. The phosphorus nutrition of tropical forage
legumes. IN: C. S. Andrew and E. J. Kamprath (eds.) Mineral nutrition of
legumes in tropical and subtropical soils. CSIRO, Melbourne, Australia. (In
press).
Armiger,
W. H., C. D. Foy, A. L. Fleeting and B. E. Caldwell. 1968. Differential
tolerance of soybean varieties to an acid soil high in exchangeable aluminum.
Agron. J. 60:67‑70.
Asher,
C. J. and D. G. Edwards. 1978. Relevance of dilute solution culture studies to
problems of low fertility tropical soils. IN: C. S. Andrew and E. J.
Kamprath (eds.) Mineral nutrition of legumes in tropical and subtropical soils.
CSIRO, Melbourne, Australia. (In press).
Baskett,
A. C. 1952. The resistance of Bact. lactis aerogenes to proflavine. II. The
direct induction of adaptation. Proc. Roy. Soc. London Ser. B 895:251‑262.
Becking,
J. H. 1961. Studies on the nitrogen‑fixing bacteria of t.. genus Beijerinckia
II. Mineral nutrition and resistance to high levels of certain elements in
relation to soil type. Plant and Soil 14:297‑322.
Bergersen,
F. J. 1961. Growth of Rhizobium in synthetic media. Aust. J. Biol. Sci.
14:349‑360.
Bergersen,
F. J. 1971. Biochemistry of symbiotic nitrogen fixation in legumes. Ann. Rev.
Plant Phys. 22:121‑140.
Brock,
T. D. 1974. Biology of microorganisms. Prentice‑Hall Inc., Englewood
Cliffs, New Jersey.
Brockwell,
J., S. K. Auso and G. A. Rea. 1966. Acid production by rhizobia from the genera
Trifolium and Lotus. J. Aust. Inst. Ag. Sci. 32:295‑297.
Broughton,
W. J., P. Y, Chan, S. Padmanabhan, and K. P. Tan. 1975. Rhizobia in tropical
legumes. I. Some characteristics of Malaysian rhizobia. Malaysian Agric. Res.
4:141‑153.
Bryan,
U. C. 1923. Effect of acid soils on nodule forming bacteria. Soil Sci. 15:37‑40.
Carvalho,
M. M. de. 1978. A comparative study of the responses of six Stylosanthes
species to acid soil factors with particular reference to aluminum. Ph.D.
Thesis. University of Queensland, Australia.
Chatel,
D. L., R. M. Greenwood, and C. A. Parker. 1968. Saprophytic competence as an
important character in the selection of Rhizobium for inoculation. Int.
Cong. Soil Sci. Trans. 9th II:65‑73.
Damirgi,
S. M., L. R. Fredrick, and I. C. Anderson. 1967. Serogroups of Rhizobium
japonicum in soybean nodules as affected by soil types. Agron. J. 59:10‑12.
Dart,
P. 1977. Infection and development of leguminous nodules. IN: R. W. F.
Hardy and W. S. Silver (eds.) A treatise on dinitrogen fixation. Section III:
Biology. John Wiley and Sons, New York, p. 367‑472.
Dean,
A. C. R, and Sir Cyril Hinshelwood. 1953. Observations on bacterial adaptation.
IN: R. Davies and E. F. Gale (eds.) Adaptation in microorganisms. 3rd
Symposium of the Soc. for Gen. Micro., Univ. Press, Cambridge, England, p. 21‑45.
Dobereiner,
J. 1966. Manganese toxicity effects on modulation and nitrogen fixation of
beans in acid soils. Plant and Soil 24:153‑166.
Dobereiner,
J and S. Andonovich. 1965. The effects of soil liming and soil temperature on
fixation of nitrogen of Centrosema pubescens, Benth., on soil
with manganese toxicity. Proc. 9th Int. Grasslands Congress, 2:1121‑1124.
(In Portugese, English summary).
Dobereiner,
J., N. E. Arruda, and A. de F. Pentado. 1965. Problems of soybean inoculation
in acid soil. Proc, of the 9th Int. Grasslands Congress, 2:1153‑1157.(In
Portugese, English summary).
Doudoroff,
M. 1940. Experiments on the adaptation of Escherichia coli to
sodium chloride. J. Gen. Physio. 28:585.
Fox, R. L. 1978. Studies on phosphorus nutrition in
the tropics. IN: C. S. Andrew and E. J. Kamprath (eds.) Mineral
nutrition of legumes in tropical and subropical soils. CSIRO, Melbourne,
Australia, (In press).
Fox, R. L., R. K. Nishimoto, J. R. Thompson and R.
S. de la Pena. 1974. Comparative external phosphorus requirements of plants
growing in tropical soils. Tran. 10th Int. Con&. Soil Sci., Moscow, IV:232‑239.
Foy, C. D. 1974. Effects of aluminum on plant
growth. IN: E. W. Carson (ed.) The plant root and its environment. Univ.
Press of Virginia, Charlottesville.
Foy,
C. D, and J. C. Brown. 1964. Toxic factors in acid soils: II. Differential A1
tolerance of plant species. Soil Sci. Soc. Am. Proc. 2:27‑32.
Foy, C. D., A. L. Fleming, and W. H. Armiger. 1969.
Aluminum tolerance of soybean varieties in relation to calcium nutrition.
Apron. J. 61: 505‑511.
Foy, C. D, and G. C. Gerloff. 1972. Response of Chlorella
pyrenoidosa to Aluminum at low phi. J. Phycol. 8:268‑271.
Fred,
E. B. and A. Davenport. 1918. Influence of reaction on nitrogenassimilating
bacteria. J. Ag. Res. 14:3I7‑336.
Frink,
C. R. and M. Peech. 1962. Determination of aluminum in soil extracts. Soil Sci.
93:317‑324.
Gillman,
G. P. and L. C. Bell. 1978. Soil solution studies on weathered soils from
tropical north Queensland. Aust. J. Soil Res. 16:67‑77.
Graham,
P. H. and C. A. Parker. 1964. Diagnostic features in the characterization of
root nodule bacteria of legumes. Plant and Soil 20:383‑396.
Graham,
P. H. and D. H, Hubbell. 1975. Soil‑plant‑Rhizobium interaction
in tropical agriculture. IN: E. Bornemisza and A. Alvarado (eds.) Soil
management in tropical America. Soil Sci. Dept. N. Carol. State Univ., Raleigh,
NC.
Ham, G. E., L. R. Fredrick and I. C. Anderson. 1971.
Serogroups of Rhizobium japonicum in soybean nodules sampled in
Iowa. Apron. J. 63: 69‑72.
Helyar,
K. R. 1978. Effects of aluminum and manganese toxicity on legume growth. IN:
C. S. Andrew and E. J. Kamprath (eds.) Mineral nutrition of legumes in tropical
and subtropical soils. CSIRO, Melbourne, Aust. (In press).
Holding,
A.. J. and J. King. 1963. The effectiveness of indigenous populations of Rhizobium
trifolii in relation to sail factors. Plant and Soil 18:191‑198.
Holding,
A. J. and J. F. Lowe. 1971. Some effects of acidity and heavy metals on the
Rhizobium‑leguminous plant association. IN: T. A. Lie and E. G.
Mulder (eds.) Biological nitrogen fixation in natural and agricultural
habitats. Plant and Soil Spec. Vol., p. 153‑166.
Hsu, P. H. 1965. Fixation of phosphate by aluminum
and iron in acidic soils. Soil Sci. 99:398‑402.
Humphrey,
B. A, and J. M. Vincent. 1952. Calcium in cell walls of Rhizobium trifolii.
J. Gen. Micro. 29:557‑561.
Jackson,
S. and Sir Cyril Hinshelwood. 1959. An investigation of the nature of certain
adaptive changes in bacteria. Proc. Roy. Soc. London Ser. B 885:562‑576.
Jensen,
H: L. 1942. Nitrogen fixation in leguminous plants. I. General characters of
root‑nodule bacteria isolated from species of Medicago and Trifolium in
Australia. Proc. Linn. Soc. N.S.W. 67:98‑108.
Jensen,
H. L. 1943. Nitrogen fixation in leguminous plants. IV: Influence of reaction
on formation of root nodules in Medicago and Trifolium. Proc. Linn. Soc. N.S.W.
68:207‑220.
Jensen,
H. L. 1947. Nitrogen fixation in leguminous plants. VII: The N‑fixing
activity of root nodule tissue in Medicago and Trifolium. Proc. Linn. Soc.
N.S.W. 72:265‑291.
Jensen,
H. L. 1969. The distribution of lucerne and clover rhizobia in agricultural
soils in Denmark. Tiddskr. Pianteavl. 73:61‑72. (In Danish, English summary).
Jones,
D. G. 1966. The contribution of white clover to a mixed upland sward. II.
Factors affecting the density and effectiveness of Rhizobium trifolii.
Plant and Soil 24:250‑260.
Jones,
D. G. and A. C. Burrows. 1969. Acid production and symbiotic effectiveness in Rhizobium
trifolii. Sail Biol. Biochem. 1:57‑61.
Jordan,
D. C, and 0. N. Allen. 1975. Rhizobiaceae. IN: R. G. Buchanan and N. G.
Gibbons (eds.) Bergeys manual of determinative bacteriology. 8th edition.
Williams and Wilkins Co., Baltimore, Md., p. 2v1‑2o4.
Kamata,
E. 1962. Morphological and physiological studies on nodule formation in
leguminous crops. 7. Variation in nodule forming capacity in strains of Rhizobium
japonicum Proc. Crop Sci. Soc. Japan 31:78‑82. (In Japanese,
English summary).
Kamprath,
E. J. 1972. Soil acidity and liming. IN: Soils of the humid tropics.
Natl. Acad. Sci., Washington, D. C.
Kamprath,
E. J. 1973. Phosphorus. IN: A review of soils research in tropical Latin
America. P. A. Sanchez (ed.) N.C. Ag. Ex. Stet. Tech. Bull. No 219.
Katznelson,
H. 1940. Survival of Azotobacter in soil. Soil Sci. 49:21‑35.
Lie,
T. A: 1971. Symbiotic nitrogen fixation under stress conditions. IN: T.
A. Lie and E. G. Mulder (eds.) Biological nitrogen fixation in natural and
agricultural habitats. Plant and Soil Spec. Vol., p. 117‑ 127.
Lie, T. A. 1974. Environmental effects on nodulation
and symbiotic nitrogen fixation. IN: A. Quispel (ed.) The biology of
nitrogen fixation. North‑Holland Publ. Co., p. 555‑582.
Loneragan,
J. F. and E. J. Dowsing. 1958. The interaction of Ca and H ions in the
nodulation of subterranean clover. Aunt. J. Agric. Res. 9:464472.
Lowther,
W. L. and J. F. Loneragan. 1970. Calcium in the nodulation of legumes. Proc, of
the 11th Int. Grasslands Cong., p. 446‑450.
Lund,
Z. F. 1970. The effect of calcium and its relation to several cations in
soybean root growth. Soil Sci. Soc. Amer. Proc. 34:456‑459.
Luria,
S. E. 1960. Bacterial protoplasm:composition and organization. IN: T. C.
Gunsalus and R. Y. Stanier (eds.) The bacteria. Vol. T9 p. 15. Academic Press,
New York.
Masterson,
C. L. 1968. Effects of some soil factors on R. trifolii. Traps.
9th Int. Cong. Soil Sci. II:95‑102.
Morris,
H. D. 1948. The soluble manganese content of acid soils and its relation to the
growth and manganese content of sweet clover and lespedeza. Soil Sci. Soc.
Amer. Proc. 13:362‑371.
Morris,
H. D. and W. H. Pierre. 1949. Minimum concentrations o£ manganese necessary for
injury to various legumes in culture solutions. Apron. J. 43:107‑112.
Mulder,
E. G. and W. L. Van Veen. 1960. Effect of pH and organic compounds on nitrogen
fixation by red clover. Plant and Soil 13:91‑113.
Mulder,
E. G., T. A. Lie, K. Dilz and A. Hauwers. 1966. Effect of pH on symbiotic N
fixation of some leguminous plants. 9th Int. Con&. for Microb. Symposia.,
p. 133‑149.
Munns,
D. N. 1965x. Soil acidity and growth of a legume. I. Interaction of lime with N
and P on growth of Medicago sativa L. and Trifolium subterraneum
L. Aust. J. Agr. Res. 16:773‑741.
Munns,
D. N. 1965b. Reactions of Al and P in solution, and effects of Al, P, Ca, and
pH on M. sativa and T. subterraneum in solution culture.
Aust. J. Agr. Res.16:74‑755.
Munns,
D. N. 1968. Modulation of Medicago sativa in solution culture. I.
Acid‑sensitive steps. Plant and Soil 2.8:129‑146.
Munns,
D. N. 1977a. Mineral nutrition gild the legume symbiosis. IN: R. W. F.
Hardy and A. H. Gibson (ells.) Treatise on dinitrogen fixation. Section IV:
Agronomy and Ecology. John, Wiley and Sons, New York, p. 353‑392.
Munns,
D. N. 1977b. Soil acidity and related matters. IN: J. M. Vincent, A. S.
Whitney and J. Bose (eds.) Exploiting the legume‑Rhizobium
symbiosis in tropical agriculture. Univ. Hawaii Coll. Trop. Agr. Misc. Publ.
145., p. 211‑236.
Munns,
D. N. 1978. Soil acidity and nodulation. IN: C. S. Andrew and E. J.
Kamprath (ells.) Mine‑wo.l,nutrition of legumes in tropical and
subtropical soils. CSIRO, Melbourne, Aust. (In press).
Munns,
D. N., H. H. Keyser, V. W. Fogle, J. S. Hohenberg, T. L. Righetti, D. L.
Lauter, M. G. Zaroug, K. L. Clarkin and K. W. Whitacre. 1978. Tolerance of soil
acidity in symbioses of Vigna radiata with rhizobia. (Submitted to
Agronomy Journal).
Norris,
D. 0. 1959. The role of calcium and magnesium in the nutrition of Rhizobium.
Aust. J. Agric. Res. 10:651‑698.
Norris,
D. 0. 1965. Acid production by Rhizobium: A unifying concept. Plant and
Soil 22:143‑166.
Norris,
D. 0. 1973. Seed pelleting to improve modulation of tropical and subtropical
legumes. 5. The contrasting response to lime pelleting of two Rhizobium strains
on Leucaena lecocephla. Aust. 3. Lip. Agr. An. Hus. 13:98‑101.
Norvell,
W. A. 1972. Equilibria of metal chelates in soil solution. IN: J. J.
Mortvedt, P. M. Giordano, and W. L. Lindsay (ells.) Micronutrients in
agriculture. Soil Sci. Soc. AM., Madison, Wisc., p. 115‑138.
Nutman,
P. S. and G. J. S. Ross. 1969. Rhizobium in the Rothamsted and Woburn
Farms. Rotramsted Lip. Sta. Rep. 2:148‑167.
Parker,
C. A. 1968. On the evolution of symbiosis in legumes. Festskrift til Hams Lauritz
Jensen. Gadgaard Nielsens Bogtrykkeri, Lemvig, Denmark, p. 107‑116.
Parker,
C. A. 1971. The significance of acid and alkali production by rhizobia on
laboratory media. Proc. 4th Aust. Legume Modulation Conf. Paper No. 10.
Canberra, Australia.
Parker,
C. A., M. J. Trinik, and D. L. Chatel. 1977. Rhizobia as soil and rhizosphere
inhabitants. IN: R. W. F. Hardy and A. H. Gibson (eds.) Treatise on
dinitrogen fixation. Section IV: Agronomy and Ecology. John Wiley and Sons, New
York, p. 311‑352.
Pearson,
R. W. 1975. Soil acidity and liming in the humid tropics. Cornell International
Agric. Bull. 30. Ithaca, New York.
Pearson,
R. W. and F. Adams. 1967. Soil acidity and liming. Agronomy No. 12. Amen. Soc.
Agron., Madison, Wisc.
Peterson,
H. B, and T. H. Gooding. 1941. The geographic distribution of Azotobacter and Rhizobium
meliloti in Nebraska soils. Univ. Neb. Coll. Agr. Res. Bull No. 121.
Probert,
M. E. 1978. Availability of phosphorus and sulphur in tropical soils in
relation to legume growth. IN: C. S. Andrew and E. J. Kamprath (eds.)
Mineral nutrition of legumes in tropical and subtropical soils. CSIRO,
Melbourne, Aust. (In press).
Reisenauer,
H. M. 1966. Mineral nutrients in soil solution. IN: P. L. Altman and D.
W. Dittman (ads.) Environmental Biology. Fed. Amer. Soc. Exp. Biol., Bethesda,
Maryland, p. 5137‑508.
Rerkasem,
B. 1977. Differential sensitivity to soil acidity of legume‑Rhizobium
symbioses. Ph.D. Thesis. University of W. Australia.
Robson,
A. D. 1978. Mineral nutrients limiting nitrogen fixation in legumes. IN:
C. S. Andrew and E. J. Kamprath (eds.) Mineral nutrition of legumes in tropical
arid subtropical soils. CSIRO, Melbourne, Aust. (In press).
Robson,
A. D. and J. F. Loneragan. 1970a. Nodulation and growth of Medicago truncatula
on acid soils. Aust. J. Agric. Res. 21:427‑445.
Robson,
A. D. and J. F. Loneragan. 1970b. Sensitivity of annual Medicago species to
manganese toxicity as affected by Ca and pH. Aust. J. Agric. Res. 21:223‑232.
Sanchex,
P. A. 1976. Properties and management of soils in the tropics. John Wiley and
Sons, New York.
Sartain,
J. B. and E. J. Kamprath. 1975. Effect of liming a highly Al-saturated soil on
the top and root growth and soybean nodulation. Agron. J. 67:507‑510.
Souto,
S. M. and J. Dobereiner. 1969. Manganese toxicity in tropical forage legumes.
Pesq. Agropec. Bras. 4:129‑138. (In Portugese, English summary).
Spencer,
D. 1950. The effect of Ca and soil pH on modulation of T. subterraneum
L. clover on a yellow padsol. Aust. J. Agric. Res. 1:374‑381.
Stranier,
R. Y. 1953. Adaptation, evolutionary and physiological. IN: R.
Davies and E. F. Gale (eds.) Adaptation :in micro‑organisms. arc:
Symposium of the Soc. for Gen. Micro., Univ. Press, Cambridge, England, p. 1‑20.
Stanier, R. Y., E. A. Adelberg and J. L. Ingraham. 1976. The
microbial world. Prentice‑Hall, Inc. Englewood Cliffs, New Jersey.
Steel,
R. G. D. and 3. H. Torrie. 1960. Principles arid procedures of statistics.
McGraw‑Hill Book Co., Inc., New York.
Steinborn,
J, and R. J. Roughley. 1975. Toxicity of sodium and chloride ions in Rhizobium
spp. in broth and peat culture. J. Appl. Bact. 39:133‑138.
Spain,
J. M. 1975. Forage potential of allic soils of the humid lowland tropics of
Latin America. IN: E. C. Doll and G. 0. Mott (eds.) Tropical forages in
livestock production systems. Amer. Soc. Agron. Spec. Publ. 24. Amer. Soc.
Agron., Madison, Wisconsin.
Truesdell,
H. W. 1917. The effect of phosphorus on alfalfa and alfalfa bacteria. Soil Sci.
3:77‑98.
Van
Schreven, D. A. 1972. On the resistance of effectiveness of Rhizobium trifolii
to a low pH. Plant and Soil 37:49‑55.
Vincent,
J. M. 1958. Survival of the root nodule bacteria. IN: E. G. Hallsworth
(ed.) Nutrition of the legumes. Proc. of Univ. of Nottingham 5th Easter School
in Ag. Sci. Butterworth Sci. Publ., London, p. 108‑123.
Vincent,
J. M. 1962. Influence of calcium and magnesium on growth of Rhizobium.
J. Gen. Micr. 28:653‑653.
Vincent,
J. M. 1965. Environmental factors in fixation of nitrogen by the legume. IN:
W. Bartholomew and F. E. Clark (eds.) Soil nitrogen. Agron. Monograph No. 10.
Amen. Soc. Agron., Madison, Wisconsin, p. 384. 435.
Vincent,
J. M. 1970. A manual for the practical study of the root‑nodule bacteria.
I.B.P. Handbook No. 15. Blackwell Sci. Publ., Oxford, England.
Vincent,
J. M. 1974. Root‑nodule symbioses with Rhizobium. IN: A.
Quipsel (ed.) The biology of nitrogen fixation. Frontiers of Biology Vol. 33.
North‑Holland Publ. Co., Amsterdam, p. 265‑34i.
Vincent,
J. M. and L. W. Waters. 1954a. The root‑nodule bacteria as factors in
clover establishment in the red basaltic soils of the Lismore District, N.S.W.
II. Survival and success of inocula in laboratory trials. Aust. J. Agric. Res.
5:61‑76.
Vincent,
J. M. and L. W. Waters. 1954b. The root‑nodule bacteria as factors in
clover establishment. in the red basaltic soils of the Lismore District N.S.W.
III. Field inoculation trials. Aust. J. Agric. Res. 5:77‑89.
Vose,
P. B. and D. G. Jones. 1963. The interaction of manganese and calcium on
nodulation and growth in varieties of Trifolium repens. Plant and
Soil 18:372‑401.
Watanabe,
F. S. and S. R. Olsen. 1965. Test of an ascorbic acid method for determining
phosphorus in water and NaHCO3 extracts from soil. Soil Sci. Soc.
Amer. Proc. 29:677‑678.
Werner,
D. and K. Berghauser. 1976. Discrimination of R. japonicum, R.
lupini, R. trifolii, R. leguminosarum and of
bacteroids by uptake of 2‑ketoglutaric acid, glutamic acid and phosphate.
Arch. Microb. 107:257‑262.
Whiting,
A. L. 1923. Inorganic substances, especially aluminum, in relation to the
activities of soil microorganisms. J. Amer. Soc. Agron. 15:277‑289.
Wilson,
D. 0, and H. M. Reisanauer. 1970. Effect of manganese and zinc ions on growth
of Rhizobium. J. Bact. 102:729‑732.
Wright,
W. H. 1925. The nodule bacteria of soybeans. I. Bacteriology of strains. Soil
Sci. 20:95‑130.











































