EFFECT OF SOYBEAN - MAIZE
CROPPING ROTATION ON SOYBEAN
RHIZOBIAL POPULATION AND
SOYBEAN NODULATION
BY
CHARLES NKWIINE
B.Sc. (Agric.) (MAK)
A THESIS SUBMITTED IN
FULFILLMENT OF
THE REQUIREMENTS FOR THE
DEGREE OF
MASTER OF SCIENCE
OF MAKERERE UNIVERSITY
DEPARTMENT OF SOIL
SCIENCE
FACULTY OF AGRICULTURE
AND FORESTRY
1990
DECLARATION
This
thesis is my original work
and
has not been presented for
a
degree in any other University.
CHARLES
NKWIINE
B.Sc.
(Agric.) M.U
This
thesis has been approved by
me
(University Supervisor) to
have
met the examiners
requirements
for M.Sc. award.

PROFESSOR
JULIUS Y.K. ZAKE
B.Sc.
(Soil Sci.) Michigan
State
Univ.
M.Sc.(Soil
Sci.)
State
Univ.
Ph.D.
Ohio State Univ. U.S.A.
DEDICATION
All
glory be
to
Almighty God
ACKNOWLEDGEMENTS
This study was carried
out at the International Institute of Tropical Agriculture (IITA), Ibadan,
Nigeria, I acknowledge the assistance given me by the Institute and its staff.
Sincere thanks are
extended to Professor Julius Y.K. Zake my University Supervisor, and Dr. K.
Mulongoy, the IITA Advisor, for their advise and support during the planning
and Conduct of the research. Dr. M.W.
Ogenga-Latigo patiently encouraged and guided me in the preparation of this
thesis and I am very grateful for his help.
Note of acknowledgements are also expressed to Dr. G.P. Msumali of
Morogoro University, Tanzania, Dr. S. Kyamanywa and Dr. E.N. Sabiiti who read
and criticized the first scribbles of the thesis and Dr. R.L. Adupa for his
guidance in the statistical analysis.
I would also like to
register my deep appreciation for the support I received from my late father
Mr. Eliasaph Bwesigye and my mother Mrs. Jairesi Nyira Bwesigye. I also thank most sincerely Messrs. H.
Bainomugisha of Mbarara, V. Lubega of Agip - Uganda, J. Tumwesigye of U.C.T.U.
and my colleagues at Makerere; T. Wanakwanyi, D. Katwire, S. Tenywa, M. Tenywa
and M. Silver for their encouragement and financial support.
The study was financed through
a research fellowship granted me by the IITA, and by a support grant by NifTAL
Project, University of Hawaii, through the intervention of Dr. P. Singleton.
I am also most grateful
to the staff of Kabanyolo Hostel for their domestic services, and Mr. Sam
Mubiru who patiently and most skillfully typed the thesis.
Finally, I wish to
express my sincere gratitude to all my brethren in the Lord Jesus Christ for
their kindness, support and constant prayers.
May the Lord bless all of
you.
ABSTRACT
Experiments were
conducted to determine the effects of soybean-maize cropping sequences on
infectiveness of introduced Bradyrhizobium japonicum and soybean
rhizobial population in the soil.
Streptomycin resistant
mutant of B. japonicum strain IRj 2114 was developed, tested for
ability to nodulate and fix nitrogen, and introduced on soybean seeds at the
start of the field experiments. Soybean (Glycine max L.) and
maize (Zea mays L.) were then grown in four cropping sequences
namely: (i) soybean/soybean/soybean (SSS), (ii) soybean/soybean/maize (SSM),
(iii) soybean/maize/soybean (SMS) and (iv) soybean/maize/maize (SMM). A glasshouse study was also carried out to
determine the effects of soybean, maize and fallowing on the survival and
establishment of rhizobia under controlled conditions.
Percentage soybean
nodulation by the introduced rhizobium, and seasonal changes in soybean
rhizobial populations along (AR) and between (BR) crop rows were determined
using the antibiotic resistance and most probable number (MPN) methods,
respectively.
Results obtained showed
that the streptomycin resistant B. japonicum nodulated
effectively and fixed a large quantity of nitrogen (158 mg/plant) in symbiosis
with soybean.
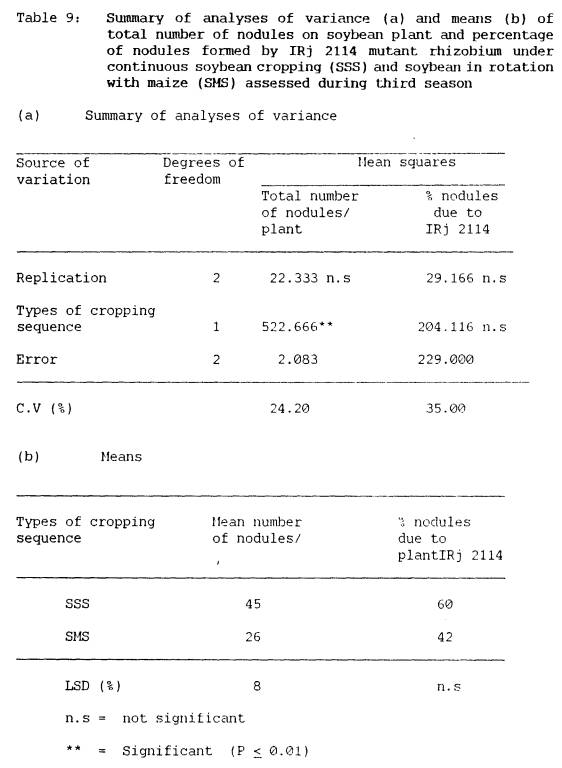
Data on soybean
nodulation showed that both the total number of nodules per plant and the
proportion of nodules due to inoculum IRj 2114 rhizobium varied significantly
(P < 0.01) with cropping sequences.
In the first season, nodule recovery due to the introduced Rhizobium was
low being only 15%. In the subsequent seasons,
maize crop adversely affected soybean nodulation. In the third season, occupancy of mutant Rhizobium which was 60%
for continuous soybean cropping (SSS) was only 42% for the soybean-maize
rotation (SMS).
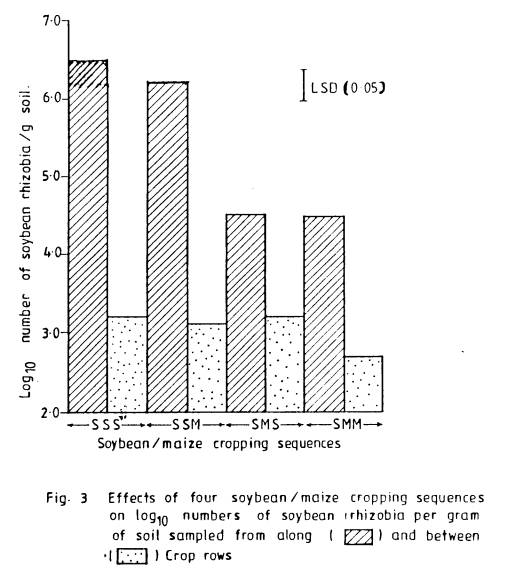
Population of soil
rhizobia were similarly affected by the cropping sequences. Rhizobial numbers were significantly (P <
0.05) higher when the first two crops were soybean (SS) than when maize
followed soybean (SM).
Throughout the sampling
period, more rhizobia occurred along the crop rows (AR) than in the inter-row
spaces (BR), indicating positive effects of rhizospheres on the rhizobial
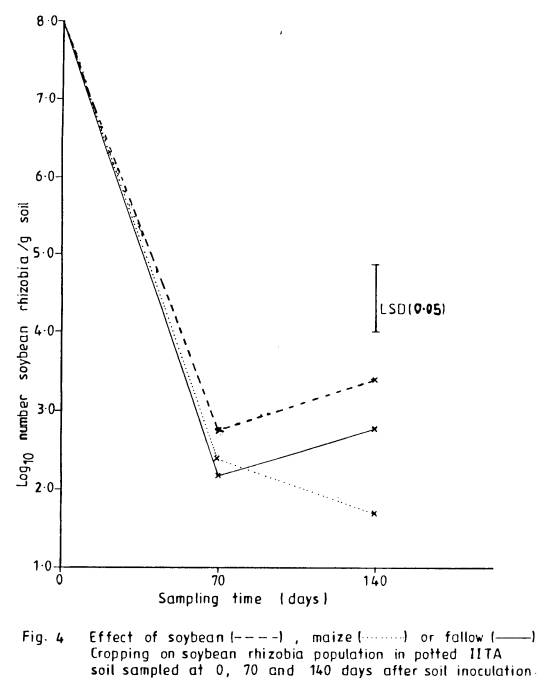
population. Pot experiment confirmed
observations in the field. Greater
stimulation of rhizobia was obtained for soybean than for maize.
It was concluded therefore
that for successful establishment of improved strains of B. japonicum, a second
soybean crop should follow the first inoculated crop.
TABLE OF CONTENTS
Page
TITLE PAGE
................................................... 1
DECLARATION .................................................. 2
DEDICATION
................................................... 3
ACKNOWLEDGEMENTS
.............................................
4
ABSTRACT
..................................................... 6
LIST OF TABLES
............................................... 12
LIST OF FIGURES
..............................................
14
LIST OF APPENDICES
...........................................
15
CHAPTER 1: INTRODUCTION
................................ 16
1.0 Introduction
................................ 16
1.1 Grain legumes and their importance
in Agriculture
.............................. 16
1.2 Importance of nitrogen fixation by
legumes
.....................................
17
1.3 The
objective of the study ..................
20
CHAPTER 2: LITERATURE REVIEW
........................... 21
2.1 Introduction
................................ 21
2.2 Biological
nitrogen fixation (BNF) .......... 21
2.3 Taxonomy
of rhizobia ........................ 22
2.4 Soil-plant-rhizobia
relationship ............ 23
2.4.1 Host-plant infection and nodule
formation
................................... 26
2.4.2 Nutrient
requirement for nodulation ......... 27
2.4.3 Senescence of nodules and release
of rhizobia into the soil
................... 28
2.5 Factors influencing rhizobial population
in
soil
........................................
28
2.5.1 Importance of soil temperature levels
... 29
2.5.2 Importance
of soil moisture levels ...... 29
2.5.3 Importance of pH and nutrient of status
of the soil
............................. 31
2.5.4 Importance
of microbial factors ......... 32
2.6 The importance of soybean rhizobia in
tropical agriculture
.................... 33
2.6.1 Response of soybean to inoculation with
rhizobia
................................ 33
2.6.2 The survival of introduced rhizobia in
soybean cereal rotations
................ 34
CHAPTER 3: GENERAL MATERIALS AND METHODS
........... 36
3.1 Location
of experiments ................. 36
3.2 Soil
types and fertilizers used ......... 37
3.3 Soybean
and maize cultivars used ........ 38
3.4 The
soybean rhizobia used ...............
39
3.4.1 Development of a culture of spontaneous
mutant of IRj 2114
...................... 39
CHAPTER 4: GLASSHOUSE
EVALUATION OF MUTANT IRj 2114
RHIZOBIUM FOR NODULATION
AND NITROGEN
FIXATION
................................ 42
4.1 Introduction
............................ 42
4.2 Materials
and methods ................... 42
4.2.1 Evaluation of nodulation, dry matter
production and nitrogen
contents of
inoculated spybean plants
............... 44
4.3 Results
................................. 44
4.3.1 Nodulation of inoculated and
uniculated soybean
...................... 45
4.3.2 Shoot dry matter yields and nitrogen
content in soybean shoots
............... 47
4.4 Discussion
.............................. 51
CHAPTER 5: EFFECT
OF SOYBEAN-MAIZE CROPPING
SEQUENCES ON THE
INFECTIVENESS OF
INTRODUCED B. JAPONICUM
AND ON
POPULATION OF SOYBEAN
RHIZOBIA .......... 53
5.1 Introduction
............................ 53
5.2 Materials
............................... 54
5.2.1 Effect of cropping sequences on soybean
nodulation and on
population of soybean
rhizobia in the field
................... 54
5.2.1.1 Establishment of field experiment ....... 54
5.2.1.2 Assessment of soybean nodulation
........ 56
5.2.1.3
Enumeration of soil rhizobia using
the "Most Probable Number"
(HPN) technique 49
...................... 58
5.2.1.3.1 Sampling of field soil ............... 58
5.2.1.3.2 Preparation of growth
pouches and
establishment of
test plants ......... 59
5.2.1.3.3 Inoculation of plants
and enumeration
of rhizobia ..........................
61
5.2.2 Glasshouse evaluation of the establishment
of soybean rhizobia in fallow roil and in
soils planted with soybean or maize in
soybean or maize
........................ 63
5.2.2.1 Enumeration of soil rhizobia using the
MPN technique ........................... 64
5.3 Results
................................. 64
5.3.1 Nodulation of soybean under different
soybean-maize cropping
sequences in
field
................................... 64
5.3.2 Population of soybean rhizobia under
different soybean-maize
cropping
sequences in the field
.................. 68
5.3.3 Population of soybean rhizobia in fallow
soil, soybean and maize
cropping in the
glasshouse
.............................. 72
5.4 Discussion
.............................. 74
CHAPTER 6: GENERAL DISCUSSION AND CONCLUSION
....... 79
REFERENCE
............................................... 82
APPENDICES
..............................................
99
LIST OF TABLES
Table Page
1 Estimates of
nitrogen fixation by various
legumes 4
.........................................
19
2 Some of the
cross-inoculation groups within
the Leguminosae
................................... 24
3 Species of the
general Rhizobium and
Bradyrhizobium and their respective
host .......... 25
4 Analysis of
variance (a) and means (b) on
nodules from test plants
grown under 2 growth ..... 46
5 Analysis of (a)
and means (b) of dry weight of
individual nodule (mg)
from test plants grown
under 2 growth
conditions, 7 weeks after planting . 48
6 Analysis of
variance (a) and means (b) of dry
shoot weight (g) from
test plants grown under 3
growth conditions, 7
weeks after planting ......... 49
7 Analysis of
variance (a) and means (b) of nitrogen
harvest (mg/plant) plants
grown under 3 growth
conditions, 7 weeks after
planting ................ 50
8 Summary of
analyses of variance (a) and means (b)
of total number of
nodules on soybean plants and
percentage of nodules
formed by IRj 2114 mutant
rhizobium in continuous
soybean cropping for
three seasons
.....................................
66
9 Summary of
analyses of variance (a) and means
(b) of total number of
nodules on soybean plant
and percentage of nodules
formed by IRj 2114
mutant rhizobium under
continuous soybean
cropping (SS) and soybean
in rotation with
maize (SMS) assessed
during third session ......... 67
10 Analysis of
variance (a) and means (b) of
soybean rhizobial
population per gram of
field soil sampled from along (AR) and
between (BR) crop rows after the first
soybean crop
......................................
69
11 Analysis of
variance (a) and means (b) of
soybean rhizobial
population per gram soil
from two soybean/maize
cropping sequences
sampled along (AR) and
between (BR) crop
rows
..............................................
70
12 Analysis of
variance with single degree
of-freedon comparisons
(a) and means (b) of
soybean rhizobial
population under three
orthogonal sets of
soybean/maize cropping
sequences and their
interactions with two
sampling positions
assessed after third
season
...........................................
71
13 Analysis of
variance (a), and means (b) of
soybean rhizobia counts
in potted soil,
under different croppings
and sampled at
2 different times after
soil inoculation ......... 75
LIST OF FIGURES
Figure Page
1 Field layout
showing the development of
4 soybean/maize cropping
sequences
during three seasons
.............................. 57
2 Positions from
which soil samples were
taken for the enumeration
of soybean
rhizobia obtained in
plots of differing
soybean/maize cropping
sequences .................. 60
3 Effects of four
soybean/maize cropping
sequences on log10
numbers of soybean
rhizobia per gram of soil
sampled
from along and between
crop rows .................. 73
4 Effect of
soybean, maize or fallow
cropping on soybean
rhizobia population
in potted IITA soil
sampled at 0, 70
and 140 days after soil
inoculation ............... 78
LIST OF APPENDICES
Appendices Page
1 Rhizobial
Populations, Physical and
Chemical Characteristics
of Experimental
Soils before planting
.............................. 99
2 Formulation of
Supplemental nutrients
required for rhizobial
and plant growth
and amount of solution
used per pot ................ 100
3 Composition of
Nitrogen-free nutrient
solution (Anon. 1982) .............................. 101
4 Number (M) of
rhizobial estimated by the
plant infection
(extracted from Vincent
1970)
..............................................
102
CHAPTER 1
INTRODUCTION
1.1 GRAIN LEGUMES AND THEIR IMPORTANCE IN
AGRICULTURE
The terms "grain
legumes" or "pulses" refer to leguminous plants producing dry
edible seeds (Okigbo, 1976).
Major grain legume species traditionally grown in the tropics include Vigna
unguiculata (L.) Walp (Cowpea), V. mungo (L) Hepper (black
gram), V. radiata (L.) Wilczek (green gram), Phaseolus vulgaris
(L.), (Common bean), P. lunatus (Lima beans), Cajanus cajan
(L.) (Millsp.) (Pigeon peas), Arachis hypogea (groundnut), Voandzeia subterranea (Bambara
nuts), and Cicer arientum (L.) (Chick pea). Soybeans (Glycine max (L.)
Merr.), although recently introduced into the tropics, has also gained
increasing importance all over the region (Auckland, 1970).
Generally, grain legumes
are grown as mixed, associated, relay and sole crops, and in crop rotations with
cereals and other crops. They are utilized in several forms for food, animal
feeds, soil cover and green manure (Rachie, 1977). Legumes also have special ability to grow in depleted soils and
even contribute to the improvement of soil fertility through their unique
symbiotic relationship with nitrogen fixing root-nodule bacteria.
Much effort has been made
to improve grain legume production levels.
However, average yields in most developing countries are still low when
compared to those obtained in the developed countries (FAO, 1983). For instance, soybean yields are reported to
average 240, 368, 553, 714 and 1167 kg/ha for Tanzania, Nigeria, Cameroon,
Rwanda and Uganda respectively. In contrast, yields of
the same crops recorded for United States of America (U.S.A.) and Brazil are
much higher, being in excess of 2400 kg/ha (Dunbar, 1975; Wilcox, 1987). These higher yields are attributable to the
use of advanced crop production techniques including the exploitation of
biological nitrogen fixation (BNF) technology.
Virtanen et al. (1947), for instance, reported that 60% of
nitrogen received by cultivated lands in U.S.A. is from biological nitrogen
fixation.
Biological nitrogen
fixation are carried out by free-living bacteria or blue-green algae which make
use of nitrogen by non-symbiotic means, and by bacteria in symbiotic
association with higher plants, mainly the Leguminosae.
Symbiotic association
between legume plants and some bacteria of the family Rhizobiaceae has
been of high significance in agriculture since 1888 (Burns and Hardy,
1975). Rhizobia (bacteria) infect
legume roots and cause formation of root nodules. Nitrogen fixation takes place inside the root nodules through the
action of the enzyme nitrogenase produced by the rhizobia. The rhizobia therefore provide fixed
nitrogen to the plant and, in return, the plant supplies the rhizobia with
carbohydrates, minerals and other nutrients.
1.2 IMPORTANCE OF NITROGEN FIXATION BY LEGUMES
When legumes are included
in a cropping rotation, they fix atmospheric nitrogen and contribute to the
nitrogen supply of succeeding crops (Hanson et al., 1988; Fox and
Piekielek, 1988; Chapman and flyers, 1987; Hesterman et al.,
1986). Estimates of the amount of
nitrogen fixed by various legume species indicate that legumes have the
potential to supply nitrogen for crop production (Table 1). Through nitrogen fixation, therefore, the
use of costly inorganic nitrogen fertilizers can be reduced and crop as well as
protein yields could be increased. This
is especially true in the tropics where nitrogen is the most limiting soil
nutrient, and where subsistence farmers can not afford the cost of fertilizers.
In the tropics, the use
rhizobia of has been limited mainly because effective Rhizobium strains
for introduced legumes such as soybean (Glycine max L. Merrill)
are lacking in the soil (Hamdi et al., 1973; Ashley, 1973; deSouza, 1969). Exploitation of symbiotic nitrogen of
soybean in tropical agriculture, therefore, requires inoculation of the crop
with appropriate rhizobia strains before planting (Friere, 1976). Soybean inoculation with rhizobia has been
found necessary even when using promiscuous soybean varieties that are able to
nodulate freely with native soil rhizobia (Pulver et al., 1982).
This is because indigenous
strains are ineffective or poorly effective, and new more effective strains
developed by genetic engineering and other means have to be introduced into
soils.
In order for the inoculum
rhizobia to be of long-term value in tropical crop production systems, they
must be able to survive, colonise, live saprophytically (outside the host) and
compete with
indigenous
rhizobial populations present in the soils.
The persistence of introduced soybean inoculum rhizobia in tropical
soils have, however, not been adequately investigated. Bradyrhizobium japonicum
strains that nodulate soybean, have been reported to survive well in the field
for more than 5 years, even in absence of the host legume (Nutman and Hearne,
1980). Crozat et al.
(1982) also reported that the percentage of nodules formed by the inoculum
strain increased with time indicating a permanent establishment and a high
competitive ability.
On the contrary, Hiltbold
et al. (1985) obtained rapid
increase in soybean rhizobial populations occurring during growth of soybeans
but
they realized a most
rapid decline in the populations in the year when cotton was grown after
soybean in a rotation. This implied
that soybean-cotton rotation had detrimental effects on B. japonicum
strains.
In the tropics, legumes
including soybean are usually grown in rotation with non-legumes particularly
cereals such as maize and rice (Sanchez, 1976). The work reported here, therefore, involved determination of the
effects of various soybean-maize cropping sequences on survival, colonization
and establishment of soybean rhizobia in the soil.
1.3 THE OBJECTIVES OF THE STUDY
Understanding of the
variations in rhizobial population, their saprophytic competence,
competitiveness and efficiency is
a first step in the utilization of introduced
rhizobia strains for improved production of both traditional and introduced
pasture and grain legumes including soybeans in the tropics (Obaton, 1977,
Alexander, 1977; Keya, 1977). In this study, therefore, efforts were made
to assess the ability of introduced Bradyrhizobium japonicum IRj
2114 to survive, colonize and he established in soils where soybean was grown
in various cropping sequences with maize.
The objectives of the
study were to determine the effects of soybean-maize cropping sequences on:
(a) the infectiveness of introduced Bradyrhizobium
japonicum
(strain IRj 2114),
(b) soybean rhizobial population in the soil, and
hence their nitrogen fixing potential.
CHAPTER 2
LITERATURE REVIEW
2.1 INTRODUCTION
Nitrogen is the most
limiting element in crop production.
This is because atmospheric nitrogen is highly inert (Timm, 1944) and
can only be transformed into usable forms such as ammonia after application of
energy and reducing agents. Yet, as an
essential constituent of proteins, nucleic acids and protoplasm, nitrogen is
highly required by crops and when in available form it is easily lost from the
soil.
It is possible to boost
the nitrogen status of soils by the application of commercial fertilizers
(Mughogho, 1985). However, the
manufacture of chemical nitrogenous fertilizers requires large amounts of
energy (Chatt, 1981) and high oil prices have increased the cost of production
and distribution of these fertilizers (Pimentel, 1976). As a consequence, a relatively cheap and
increasingly important source of nitrogen for the crops is that fixed by soil
micro-organisms (Quispel, 1974).
2.2 BIOLOGICAL NITROGEN FIXATION (BNF)
Biological nitrogen
fixation comprises of non-symbiotic and symbiotic systems. Non-symbiotic nitrogen fixation involves
free-living organisms like Azotobacter, Klebsiella, Clostridium
and many algae which are able to fix atmospheric nitrogen independently. Symbiotic nitrogen fixation, on the other
hand, is based on very close physical and physiological associations between
rhizobial bacteria and leguminous plants.
The bacteria fix atmospheric nitrogen by incorporating nitrogen gas from
the atmosphere into forms utilizable by legumes for the synthesis of organic
compounds.
Although non-symbiotic
organisms fix nitrogen, their contribution to the nitrogen economy of the soil
is not as great as those of the symbiotic ones (Hardy and Havelka, 1975). For example, Meiklejohn (1954) reported that
non-symbiotic nitrogen fixation ranges from 10 to 15 kg N/ha/year. In contrast, rhizobia in symbiosis with
legumes, are believed to fix nitrogen at levels varying from less than 100 kg
N/ha/year to more than 600 kg N/ha/year (Graham and Hubbell, 1975). Estimates show that the symbiotic system
contributes 40 million tons of nitrogen annually to grain legumes (Hardy and
Havelka, 1975). Rhizobia-legume
symbiosis is therefore the most important source of biologically fixed nitrogen
in agricultural systems.
2.3 TAXONOMY OF RHIZOBIA
Rhizobia are rod-shaped,
gram-negative and non-spore forming bacteria.
They are aerobic and can be found free-living in soils, or cultured in
agar (Vincent, 1982).
Systems of classification
of rhizobia have undergone many changes.
The earlier classifications were based on the "Serum zone"
(Trinick, 1982) and "Cross inoculation group" (Jensen, 1958)
concepts.
Serum zone classification
was based on the characteristic reaction that many rhizobia have when they are
grown in skim milk medium. The bacteria
produce a
superficial clear medium - the "Serum zone" - which characterizes
each species by the change in pH towards acid or alkaline. The concept has limited value in
distinguishing rhizobia because even within the homogeneous group, for example
rhizobia from Caragana arboroscens (Jensen, 1942) and Lucerne (Medicago
sativa L.) (Trinick, 1982), it is possible to find strains with
and without serum zone formation.
The cross-inoculation
group classification, on the other hand, was based on the host range of the
bacteria (Fred et al., 1932).
Within the particular "Cross-inoculation" group, rhizobia from
one plant would nodulate all other plants and vice versa. The group of rhizobia that form nodules in
each member of the cross-inoculation group were then regarded as belonging to
the same species.
This is illustrated in
Table 2. It has however, become evident
that these groups are not discrete and many reports of boundary-jumping
between them are available (Trinick, 1982; Masefield, 1958; Kleczkowska et
al., cited by Jensen, 1958).
Because the above systems
of classification have been found to be biologically inaccurate, they have been
largely discarded (Jordan, 1982). The recent concept of rhizobial
classification is based on techniques designed to examine large portions of the
bacterial genome. On this basis, Jordan
(1984) classified root-nodule bacteria under two genera namely; (i) Rhizobium
and (ii) Bradyrhizobium. Rhizobium
consists of all fast growing acid producing rhizobia while Bradyrhizobium
comprises of the slow growing alkali producing rhizobia. The corresponding rhizobia species, based on
this classification, and their hosts are given in Table 3.
2.4 SOIL-PLANT-RHIZOBIA RELATIONSHIP
Rhizobia are known to live freely in soil in the absence of
their host plants (Rovira, 1961; Vincent, 1974; Nutman and Hearne, 1980).
However, as rhizosphere
organisms, these bacteria are markedly stimulated to multiply by the root
secretion of nutrients and growth factors (West, 1939), and they rapidly
increase in number in rhizosperes (Vincent, 1982). This stimulation has been
found to be general and not confined to leguminous roots only, although the
degree of stimulation varies between non-legumes and legumes (Diatloff, 1969;
Mahler and Wollum, 1982). Generally,
non-legumes stimulate the rhizosphere root-nodule bacteria to a smaller degree
(Lockhead, 1952; Rovira, 1961).
Multiplication and
increase of rhizobia in numbers in the vicinity of legume root hairs is an
essential prerequisite for the infection process and nodulation (Bergersen,
1977). In soil or cultures, the actual
densities of nodule bacteria required in the rhizosphere for the successful
infection are of the order of 106 to 109 organisms per ml
(Purchase and Nutman, 1957).
2.4.1 Host-plant infection and nodule formation
Rhizobium forms an infection
thread toward the base of the root hair and eventually penetrates the cortex of
the root (Newcomb et al., 1979; Rao and Keister, 1978). The bacteria still continue to divide, then
later on division stops and the bacteria grow into swollen, mostly branched
cells which are called bacteriods.
Bacteriods are able to fix nitrogen through nitrogenase enzyme activity. In response, the legume plant root hair
undergoes rapid cell division of the meristematic tissue in the vicinity of the
infection and forms a tuberous growth, the nodule (Dazzo, 1980).
Occasionally, nodule-like
growths may be produced on the roots of legumes by nematodes or by crown-gall
bacteria. Certain non-leguminous plants
are also frequently found to possess nodule-like growths which are produced by
mycorrhiza crown-gall organisms and certain nematodes. On careful examination, however, Rhizobium
induced nodules are easily distinguished from the false ones.
Individual nodules vary
greatly in size and shape. For example,
the cultivated annual legumes like soybean, generally have large spherical
nodules, whereas those on the biennial and perennial legumes tend to be
smaller, elongated, and clustered.
In the nodule, rhizobia
occur in both normal vegetative rod shaped cells and as bacteriods. The bacteriods are rhizobial cells that have
differentiated and are not capable of multiplication as free cells. The coexistence of both vegetative
rhizobial cells and non-viable bacteriods was observed in nodules of Astragalus
senicus, Medicago, Trifolium and Vicia (Date and
Halliday, 1987). The vegetative cells
and bacteriods show no apparent morphological differentiation (Date and
Halliday, 1987).
2.4.2 Nutrient requirement for nodulation
Root nodules are rich in
molybdenum, phosphorus, cobalt, iron, zinc, sulphur and nitrogen (Munns, 1977;
Robson, 1978). The high concentration
of these elements in the nodules is associated with high bacteroidal
concentration of nuclceotides, cobalamines and proteins, including Fe-, S- and
Mo-proteins, and the presence of iron in legheamoglobin (Allen and Allen, cited
by Manil, 1958). Deficiencies of these
elements in the soil will affect Rhizobium legume symbiosis. It is known, for example, that symbiotic
plants need higher rates of phosphorus fertilization than nitrogen fed plants
(Cassman, 1979), and when phosphorus requirements are not satisfied, nodule
formation and functioning are adversely affected (Vincent, 1965; Olsen and Moe,
1971). Similar effects are also known for such micronutrients as molybdenum
and sulphur, which are constituents of the enzyme nitrogenase and are important
nutrients for the rhizobia (Perkasen, 1977; Munns, 1978). Thus, plants that are dependent on
symbiotically fixed nitrogen require greater quantities of macro- and
micro-nutrients than their non-symbiotic counter-parts (Jonnes and Lutz, 1971;
Burton, 1972 Robson, 1978).
2.4.3 Senescence of nodules and release of rhizobia
into the soil
Nodule death occurs
because of plant senescence or other factors like drought, high soil
temperatures and nutritional disorders that affect nodule life. The rhizobia then die or are released into
the soil, hence completing their life cycle (Sutton, 1983). Since each nodule may contain millions of
rhizobia and the number of nodules that may develop on a single plant vary from
a few to a thousand or more, decayed nodules release vast numbers of rhizobia
into the soil, thus increasing the rhizobial population.
Bushby (1981) obtained
increased number of soybean rhizobia in soils grown with the crop at 70 days
after planting. He attributed the
increase to nodule decay and the release of rhizobia into the soil. Where inoculation using effective Rhizobium
strains has to be done in the tropics, for high yielding soybean varieties
(like Bossier), therefore, rhizobia originating from nodule disintegration are
likely to form an important component of the rhizobial populations which
nodulate subsequent crops (Brockwell et al., 1988).
2.5 FACTORS INFLUENCING RHIZOBIAL POPULATION IN
THE SOIL
Besides the presence of
host and non-host plants, the persistence of free-living rhizobia in soils is
generally influenced by physical, chemical and biotic factors (Lowendorf ,
1980). The major factors include soil
temperature and moisture levels, pH and nutrient status of the soil, and the
activity of other micro-organisms.
2.5.1
Importance of soil temperature levels
Many root-nodule,
bacteria grow best under a temperature range of 25°C to 30°C
(Vincent, 1970). Most strains of genus Bradyrhizobium,
however, are reported to be tolerant to high soil temperatures with a maximum
growth range within 30-400C (Jordan, 1984). Extremes of soil temperatures, therefore,
affect the survival and persistence of rhizobia in soils.
In the tropics, high soil
temperatures are a major factor limiting the activity of rhizobia, particularly
exotic strains but also below 150C nodulation may not occur (Elkan,
1987). Studies in Ibadan, Nigeria,
showed that soil temperatures can reach an average of 40°C at
0-15 cm depth, when soils
are bare or newly planted with crops (IITA, 1972; Lal, 1975). The introduction into the tropics of
temperate strains of rhizobia, as inoculum for soybeans and other crops, that
are not tolerant of such high temperatures, will introduce the problem of their
adaptation and persistence in these soils.
The effect of high soil temperatures is made worst by the fact that such
conditions occur during the dry off-season periods when crop hosts may not be
growing in the field.
2.5.2 Importance of soil moisture levels
In the tropics, and
else-where, soil moisture conditions may range from a state of water logging to
total dryness. Because rhizobia are
aerobic heterotrophs, flooding the soil reduces the gas exchange between the soil
and bacteria or plant nodules and thus affect the growth and activity of
rhizobia. Desiccating conditions, on
the other hand, reduce available soil moisture and may lead to the death of
rhizobia (Pena-Cabriales and Alexander, 1979).
Rhizobial species vary in
their response to variation in soil moisture levels; while some are tolerant of
a wide range of moisture conditions others are not. Osa-Afiana and Alexander (1979) compared the survival of Rhizobium
trifolii and the soybean rhizobia, Bradyrhizobium japonicum,
and found
that higher numbers of both rhizobia species survived at 10% relative humidity
than at moisture regimes ranging from 22% to 45% relative humidity. They, however, also found that while the
number of surviving R. trifolii decreased with increase in soil
moisture levels, the residual population of the soybean rhizobia was higher (2%
of the original population) at the higher moisture level (45%) than at the
lower moisture levels (0.7% to 22% relative humidity).
The variable response of
the two rhizobia species was also observed under extremely high moisture
levels. Thus, when soils were flooded,
the population of R. trifolii was reduced by a factor of 300
(from 1.3 x 108 to 4.2 x 104 cells per gram of soil while
that of soybean rhizobia was reduced by a factor of 150 (from 6.0 x 108
to 4.0 x 106 cells per gram of soil) (Osa-Afiana and Alexander,
1979).
The tolerance of soybean
rhizobia of a wider range of soil moisture conditions than R. trifolii
could be of importance in the adaptability of soybean rhizobia to tropical
soils that experience large variations in soil moisture levels.
2.5.3
Importance of pH and nutrient status of the soil
Both soil pH and nutrient
levels have direct and interactive effects on the survival and multiplication
of rhizobia in the soil, the growth of legume host, and their nodulation and
nitrogen fixation. Although soil pH
levels affect both plant growth and the occurrence, survival and growth of
rhizobia, the rhizobia are more often affected by pH levels than the plants in as much as the host
can grow in soils in which these organisms perish rapidly (Loneragan and
Bowling, 1958).
Optimal pH for growth of
rhizobia is between pH 6 and 7.
However, the slow-growing and alkaline producing species such as Bradyrhizobium
japonicum are more tolerant of lower pH levels while the fast growing and
acid producing species such as R. leguminosarum are more tolerant
of higher pH levels (Graham and Parker, 1964; Wilson, 1970; Jordan, 1984; Krieg
and Holt, 1984). In the tropics, high
temperatures together with heavy rains cause rapid decomposition of organic
matter and mineral leaching especially of bases. Tropical soils are therefore mainly acidic with pH below 6
(Sanchez, 1976). Acidity directly
inhibits nodule formation, and nodulation failure in acidic soils is usually
attributed to poor survival of rhizobia or their failure to multiply in the
rhizospheres (Vincent, 1965). Low soil
pH is usually also associated with nutrient deficiency and mineral toxicity for
the rhizobia. For example, molybdenum
deficiency is common in acid soils (Munns, 1978). On the other hand, although iron deficiency is not common in the
tropics, its high solubility under acid conditions often raises its availability
to levels toxic to both rhizobia and plants.
The survival of rhizobia and legume nodulation in such tropical soils
are therefore greatly affected.
2.5.4 Importance of microbial factors
The microbes that
possibly regulate the number of rhizobia in tropical soils include predators
like protozoa and amoebae, and parasites namely: bdellovibrios and
bacteriophages. Their importance in the
regulation of rhizobial populations in the soil is still unclear.
In a study of rhizobial
predation by protozoans, Alexander (1975) reported that each protozoan consumes
about 80 soybean rhizobia cells/day.
However, the predators failed to eradicate the rhizobia because the
remaining bacterial cells were able to reproduce at a rate fast enough to
replace the cells that were consumed.
Danso and Alexander (1975)
found amoebae to prey on root-nodule bacteria at a rate of 103 to 104
cells per replication. Despite the
enormous number of rhizobia needed by the amoebae, high rhizobial population is
found to survive in soils inoculated with rhizobia.
Bdellovibrios viruses are
considered of little importance in lowering the populations of root-nodule
bacteria in field soils. This is
because the rhizobia seldomly attain population densities needed to initiate
feeding of the parasites (Keya, 1974; Keya and Alexander, 1975).
Bacteriophages are also
known to parasitise rhizobial cells (Vandecaveye et al.,
1940). However, they do not eliminate
rhizobia from the soils. The inability
of bacteriophages to eliminate root-nodule bacteria from the soil is partly due
to host specificity of the parasites (Hitcher, 1930). Furthermore, the existence of large numbers of bacteriophages in
the soil will often lead to the development of bacteriophage - resistant
rhizobia mutants (Vandecaveye and Moodie, 1943; Kleckowska, 1957).
2.6 THE IMPORTANCE OF SOYBEAN RHIZOBIA IN
TROPICAL AGRICULTURE
Soybean is an introduced
crop in the tropics. Therefore, for
high nitrogen fixation and better yields, the crop requires inoculation of
seeds with the appropriate rhizobia strains before planting (Freire,
1976). This is because there is a lack
of suitable strains of Rhizobium for soybean as has been reported on
most Uganda soils (Ashley, 1973). Similar observations have been also reported
from Egypt (Hamdi et al., 1973) and Kenya (deSouza, 1969).
2.6.1 Response of soybean to inoculation with
rhizobia
Inoculation ensures
successful symbiosis by introducing effective rhizobia strains into soils, in
the proximity of seeds, thus enhancing nitrogen fixation by legume plants. Remarkable positive response of soybean to
rhizobia inoculation have been obtained in many tropical countries. In Tanzania, for example, Bossier variety,
which failed to nodulate without inoculation, when inoculated gave an increased
yield of 300 percent (Min. Agric., Tanzania, 1978). Similarly, in experiments carried out in Nigeria using superior
strain inoculates, high yielding soybean cultivars like Bossier and TGM 294-4
showed yield increases of up to 100 percent (IITA, 1978). Contribution of seed inoculation in increasing
soybean yields has also been demonstrated by the use of most promising Malawian
strains of rhizobia on the soybean variety Gedult. Average yields of 3148 kg
seed/ha, were obtained as compared to 2703 kg/ha for the control (Anon., 1969). Studies in India by Jethmalani et al.
(1969), also showed significantly higher yields for inoculated soybean than for
noninoculated crops that received 120 kgN/ha.
Rhizobium-soybean symbiosis is of
comparable importance to other Rhizobium-legume associations with respect
to nitrogen economy of the succeeding crop.
Gomez (1968) studied soybean(s)-maize(m) cropping sequences namely m-m,
s-s, m-s and s-m. He found that maize
in rotation with soybean maintained high yields similar to those of sequential
maize fertilized with nitrogen.
Similarly, Caldwell (1982) obtained 14 percent yield increase above
nitrogen treatments for maize following soybean and attributed this to nitrogen
fixed by the soybean.
The contribution of
soybean rhizobia to soil nitrogen economy was also demonstrated in an intercrop
system by Searle et al. (1981). They
showed that nitrogen uptake by wheat following an intercrop of maize and
soybean was about twice that following maize alone without nitrogen and was equivalent
to that following maize fertilized with 100 kgN/ha. This could be of great significance in the tropics where
intercropping and crop rotations are major crop production systems (Okigbo,
1978). The long term value of rhizobial
strains introduced into soils through inoculation will, however, only be
realized if the production of soybean crops is substantially supported by
nitrogen fixed by these rhizobia.
2.6.2 The survival of introduced rhizobia in
soybean-cereal rotations
Fields in which soybeans
have been groan frequently have populations of bradyrhizobia which are normally
adequate for effective nodulation of subsequent soybean crops (Crozat et
al., 1982; Weaver et al., 1972). However, there is evidence of very low field recovery of nodules
from inoculum rhizobia.
Johnson et al.
(1965) obtained 5% of nodules from the inoculum applied at standard rate
(approximately 1x105 cells/seed).
Cardwell and Grant (1970) reported a range of 5 to 10% nodulation due to
inoculum rhizobia while Ham et al. (1971) realized 0-17% nodule
recovery from inoculum strains. These
observations indicate poor survival, colonization and establishment of inoculum
rhizobia in these soils.
Under tropical
conditions, non-indigenous legume species, such as soybean, have to be
inoculated with appropriate rhizobial strains in order for them to successfully
fix nitrogen (Freire, 1976). Because
such legumes are commonly grown in rotations with cereals namely: maize (Zea
mays L.), rice (Oryza sativa L.), wheat (Triticum aestivum
L.) and sorghum (Sorghum bicolor L.) (Okigbo, 1978; Sanchez,
1976) a major problem is the ability of the introduced rhizobia to survive
during the non-legume cropping season and so sustain high yields of a
subsequent legume crop without reinoculation.
CHAPTER 3
GENERAL MATERIALS AND METHODS
Materials and methods
outlined in this chapter are of a general nature; those specific to particular
experiments are given in the appropriate sections.
3.1 LOCATION OF EXPERIMENTS
Field and pot experiments
were carried out at the International Institute of Tropical Agriculture (IITA),
Ibadan, Nigeria, between March 1984 and June 1985. The Institute is located in the rain forest savanna transition
zone of South-western Nigeria at latitude 7° 30'N and longitude 30°
54'E, and occupies about 1000 ha. The topography
of the site is rolling with dominant slopes between 3 and 10%. The landform is that of an eroded pediment
plain, with well incised valleys forming a trellis pattern.
Moorman et al.
(1975) gives a detailed description of the climate and soils of IITA. Annual rainfall at the station is bimodal,
with peaks in June and September and a major dry season between December and
February. Total rainfall ranges from
788 mm to 1884 mm. Annual average
temperatures range from 21.30°C to 31.20°C with extreme daily
minimum and maximum temperatures of 8.30°C and 38.00°C,
respectively.
Soils of IITA have been
classified under 8 series, namely Ekiti Lwo, Egbeda, Ibadan, Gambari, Apomu,
Iregun and Matako (Moorman et al., 1975). On the basis of the FAO classification,
these soil types can be grouped as follows:
Lwo, Egbeda, Ibadan and Iregun are Ferric
Luvisols, Ekiti is an Eutric Cambisol,
Gambari is Plinthic Luvisol, Matako is Mollic Gleysol and Apomu is an Albic
Arenosol.
Field experiments were
carried out on Block A10 in an area of about 0.1 hectare in size. The soil of this block is of the Apomu
series, consisting of sandy upland and slopes which are especially drought
susceptible, with high leaching losses of applied nutrients (Moorman et al.,
1975). Because the area was partly flat
and had not been grown with soybean for over three years, it was considered
suitable for the study on soybean-maize rotation involving inoculation of
soybean with Bradyrhizobium.
3.2 SOIL TYPES AND FERTILIZERS USED
When carrying out the
first pot experiments, two soil types namely IITA and Fashola soils were
used. IITA soil was collected from
Block A10 while the other soil was collected from Fashola, located in a savanna
grassland region 70 km North of IITA.
IITA soil has a high population of native rhizobia as compared to
Fashola soil that had hardly any rhizobia (Ayanaba et al., 1981). The use of these soils, therefore, provided
contrasting ecological environments (presence or absence of native rhizobia)
for evaluation of symbiosis of the rhizobia strain used.
The soils were collected
at 0-15 cm depth. Each soil type was
mixed thoroughly and a composite sample of 1 kg taken for determination of
physical, chemical and microbial characteristics. Soil texture was determined using the hydrometer method (IITA,
1979). Chemical analysis included
determination of pH, organic carbon, total nitrogen and such minerals as
phosphorus, exchangeable potassium, calcium, manganese and aluminium (IITA, 1979;
Bremner, 1960; Walkley, 1947). Determination
was also made of the population of indigenous soybean rhizobia found
in these sails. The results of the soil
analysis are presented in Appendix 1.
Fashola soil had no soybean rhizobia and low nitrogen content and hence
the soil was suitable for evaluation of infectivity and effectiveness of
introduced mutant strain.
Based on chemical
characteristics of the soils the fertility level was low, in both field and pot
experiments, nutrients were added to the soils to raise soil fertility to
levels considered ideal for rhizobial activity (Vincent, 197O). Phosphorus was supplied as single
superphosphate at the rate of 100 kg P2O5/ha. The single
superphosphate applied was assumed to supply about 12% sulphur. Molybdenum was applied in form of sodium molybdate
at the rate of 1 kg Mo per hectare.
Potassium was supplied as muriate of potash at 6O kg K2O per
hectare.
The same applications of
K and Mo were done before every subsequent planting. Phosphorus was not applied in subsequent seasons because single
superphosphate has a good residual property (Sanchez, 1976) and substantial
quantities of P are available to subsequent crops. Application of the fertilizers was done before planting and the
fertilizers worked into the soil.
3.3 SOYBEAN AND MAIZE CULTIVARS USED
Soybean (Glycine max
L.; cultivar TGx-17-2Ge) and maize (Zea mays L.; variety
Gusau-82) were used as test crops for both field and pot experiments. Both maize and soybean varieties are local
cultivars developed
at IITA.
TGx-17-2GE is a moderately
promiscuous soybean cultivar as it is able to nodulate fairly well with
rhizobia indigenous to IITA soils (IITA, 1982). The cultivar also has high germination percentage.
The maize variety
Gusau-82 was preferred because it has almost equal maturity period and equal
inter-row spacing requirements as the soybean cultivar. This made soil sampling and other agronomic
operations easy.
3.4 THE SOYBEAN RHIZOBIA USED
Rhizobium bank of IITA
microbiology laboratory had two strains of soybean rhizobia which exhibited
differing resistance to antibiotics.
One was resistant to streptomycin and another to spectomycin. Bradyrhizobium japonicum
strain IRj 2114, resistant to streptomycin (aminoglycoside), was preferred to
the spectomycin resistant mutant. This
was because mutants which are resistant to streptomycin are more stable and
frequently do not lose their symbiotic capacity (Somasegaran and Hoben, 1985).
3.4.1 Development of a Culture of Spontaneous
mutant of Irj 2114
To recognize the inoculum
strain after it had been introduced into soil, a mutant of IRj 2114 was
developed for spontaneous resistance level of 1000 micro(μ)g/ml
streptomycin sulphate as described by Hagedorn (1979). Samples of IRj 2114 strain from selected
slant were aseptically cultured in a flask containing 50 ml of yeast mannitol
broth (YMB).
The broth was prepared by
dissolving 10.0g of mannitol; 0.5g potassium hypophosphate (K2HPO);
0.2g crystalline magnesium sulphate (MgSO·7H2O); 0.1g sodium
chloride (Nacl) and 1g yeast extract in 1 litre of distilled water. The solution was then adjusted to pH 6.8 and
autoclaved at 1210C and 718.50 pascals pressure for 30 minutes,
before cooling. The broth culture of
the rhizobium was grown on a rotary shaker (100 revolutions/min) under normal
laboratory conditions for 7 days (Vincent, 1970). Samples of the IRj 2114 strain from this culture were then
aseptically transferred onto yeast mannitol agar (YMA) plates.
Yeast mannitol agar (YMA)
was prepared by adding 3g of potato dextrose agar to 200 ml of yeast mannitol
broth (YMB) in a 500 ml Erlenmeyer flask and autoclaving as above. Ten millilitres of a solution of 400 mg of
streptomycin sulphate dissolved in 20 ml of distilled water filtered through a
sterile millipore filter of 0.2 μm pore size was then added to the YMA
maintained at 600C. The
mixture was shaken carefully to avoid the formation of air bubbles, after which
the flasks were returned to the water bath maintained at 600C for 10
minutes to re-equilibrate and allow the air bubbles to dissipate from the
agar. Approximately 200 ml of this
mixture were then poured onto a sterile petri dish to form an agar plate of
1000 μg streptomycin/ml agar.
Inoculum of the IRj 2114
streptomycin resistant strain, cultured in YMB, was aseptically spread on the
YMA plates using a sterile loop. The
plates were then incubated at 28°C for seven days in an inverted
position, there after the plates were examined for rhizobial growth.
Yeast mannitol broth
containing streptomycin (YMB-Str.) at a concentration of 1000 μg/ml was
prepared as for YMA of the plates above except that no agar was added. Two hundred ml of the YMB str. was put into
each flask.
Distinct colonies from
the plates with rhizobial growth were selected and, using a sterile loop, part
of the colony was aseptically transferred into the flasks. The
inoculated YMB-Str, was then placed on a rotary shaker for 7 days. The resulting culture contained IRj 2114
cells with spontaneous resistance to 1000 μg streptomycin/ml broth. Authentication of the culture was done using
procedures described by Vincent (1970), before use as inoculum.
CHAPTER 4
GLASSHOUSE EVALUATION OF
MUTANT IRj 2114 RHIZOBIUM FOR
NODULATION AND NITROGEN
FIXATION
4.1 INTRODUCTION
During the process of
marking rhizobial strains with antibiotics, such as streptomycin, they may lose
their ability to establish viable symbiotic relationships with the host plants
(Josey et al., 1979). On
this basis, an experiment was carried out to ascertain the ability of the IRj
2114 spontaneous mutant rhizobium to nodulate and fix nitrogen.
4.2 MATERIALS AND METHODS
The activity of the IRj
21I4 mutant was assessed in Fashola soil which had no soybean rhizobia, and was
low in nitrogen and in IITA soil, the main experimental soil that had native
soybean rhizobia. The soils were
collected and sieved through a 2 mm sieve
before pot filling. Eighteen medium
sized plastic pots (top diameter 20 cm, depth 18 cm) cure each filled with 3 kg
of the soils. Nine pots were allocated
to each soil type.
Based on chemical
analyses of the soils (Appendix 1), nutrients were added to boost their
fertility levels. Phosphorus, potassium
and molybdenum were applied as described in Chapter 3, Section 3.2. As part of the treatments, nitrogen
fertilizer was applied to soils in three pots, for each soil type at the rate
of 80 g of urea per pot. This was
equivalent to 120 kg N/ha.
Good soybean seeds were selected and surface sterilized by immersing in 0.2% HgCl for 3 minutes followed by rinsing with 95% ethanol. The seeds were then washed in 8 changes of sterilized distilled water
before inoculation with rhizobia (Vincent, 1970). Inoculation was done by
applying a heavy suspension of IRj 2114 mutant, rhizobium, cultured in yeast
mannitol broth as described in Chapter 3, Sub-Section 3.4.1 on the sterile
seeds. To improve the survival of the
rhizobia pre-sterilized peat was added to the Bradyrhizobium broth
suspension at the rate of 25 g of peat to 100 ml of broth suspension (Vincent,
1970). A boiled solution of 100 g gum
arabic in 230 ml of sterile water was also added to seeds as an adhesive at a
rate of 4 ml for about 100 seeds. The
seeds were then mixed thoroughly for 5 minutes. This process gave an inoculation rate of approximately 107
rhizobia per seed.
The inoculated and uninoculated
soybean seeds were planted in pots at the rate of 6 seeds per pot. The treatment combinations were as below:
(i) Inoculation with IRj 2114 mutant Rhizobium,
resistant to 1000 μg streptomycin/ml of broth.
(ii) No inoculation but inorganic nitrogen was
applied at the rate of 120 kg N/ha.
(iii) Control- with no inoculation and no nitrogen
application. Each combination was replicated three times.
Fourteen days after
germination, when healthy plants could be selected, seedlings were thinned to 3
plants per pot. This was the maximum
number of plants that could be maintained in each pot on 3 kg of soil up to the
time of sampling, seven weeks after planting. Immediately after thinning,
supplemental nutrients required for rhizobial and plant growth (Appendix 2)
were added in solution form by uniformly spraying and working it into the soil. All the
pots were daily watered using tap water throughout plant growth period,
4.2.1 Evaluation of nodulation, dry matter
production and nitrogen contents of inoculated and uninoculated soybean plants
To determine nodulation,
plant dry matter production and nitrogen accumulation in plant shoots, the
soybean plants were harvested 49 days after planting. Plants from each pot were carefully uprooted using a hand trowel,
and plant tops excised at crown level, placed in paper bags, oven dried at 700C
to constant weight, and the above soil surface dry matter production obtained.
The shoots were then ground to fine texture and total nitrogen content of the
shoots determined using the micro-Kjeldahl method. Nodulation was assessed by
examining the roots of individual plants, Nodules were picked from the roots,
and those that dropped off into the soil were also collected. The nodules were washed and counted and the
total number of nodules collected from each pot recorded. The nodules were then
oven dried to constant weight as above, and their oven dry weights determined.
Data collected were
subjected to analysis of variance (ANOVA) and means compared using the least
significant difference (LSD) test, at the level of P < 0.01 (Steel
and Torrie, 1960). When analyzing for
nodule per plant and dry weight of individual nodules, data for plants that
received nitrogen were excluded because of the depressive effects that nitrogen
application has on soybean nodulation (Diatloff 1967; McNeil 1982; Herridge et
al., 1984).
4.3 RESULTS
4.3.1 Nodulation of inoculated and uninoculated
soybean
Data on the nodulation of
soybeans under the different, treatment conditions are presented in Table
5. Analysis of variance of tire number
of nodules on soybean plants showed that nodulation was significantly (P <
0.01) influenced by soil type and seed inoculation with the mutant rhizobia
(Table 4a). There were also significant
(P < 0.01) interactions between the two factors.
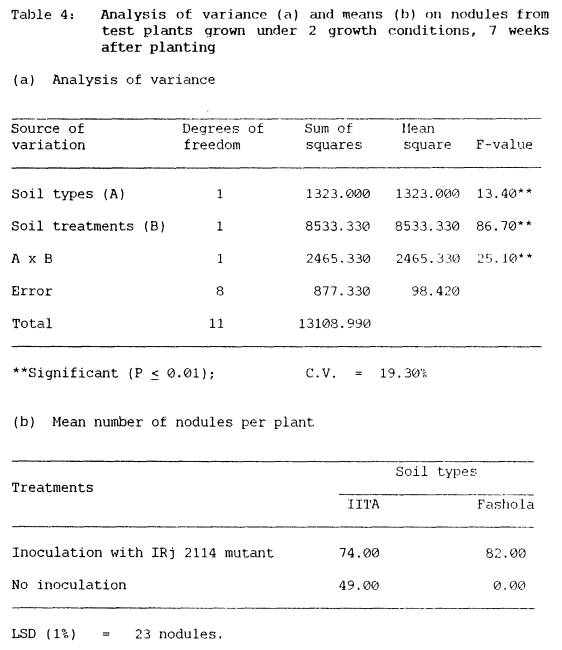
A high number of nodules
were formed by soybean inoculated with mutant.
IRj 2114 in both Fashola and IITA soils. The number of nodules on plants grown in Fashola soil were
substantially higher than that on plants grown in IITA soils. There were on average 82.0 and 74.0 nodules
per plants for the two soils, respectively.
For IITA soil, nodulation of plants inoculated with mutant rhizobia were
50% higher than uninoculated plants (Table 4b).
Mean dry weights of
individual nodules were significantly (P < 0.01) influenced by the
simple and interaction effects of soil type and soil treatment (Table 5a). Nodules formed by the IRj 2114 mutant were
smaller than those formed by the indigenous soybean rhizobia. Thus the average weight of nodules obtained
from plants inoculated with rhizobia and grown in Fashola soil was 6.9 mg while
that for uninoculated plants grown in IITA, soil was 10.4 mg (Table 5b). Observations on soybean nodulation showed
that IRj 2114 mutant rhizobia formed numerous nodules with soybeans. The nodules formed were, however, smaller
than those formed by the native rhizobia strains in IITA soil.
4.3.2 Shoot dry matter yields and nitrogen content
in soybean shoots
Analyses of dry weight of
soybean shoots and their nitrogen contents are presented in Tables 5 and 7
respectively. Both dry matter yields
and nitrogen content of plants were highest when soybeans received fertilizer
nitrogen. However, inoculation of
soybean with IRJ 2114 mutant rhizobia also effectively increased shoot dry
weight and nitrogen content of the crop.
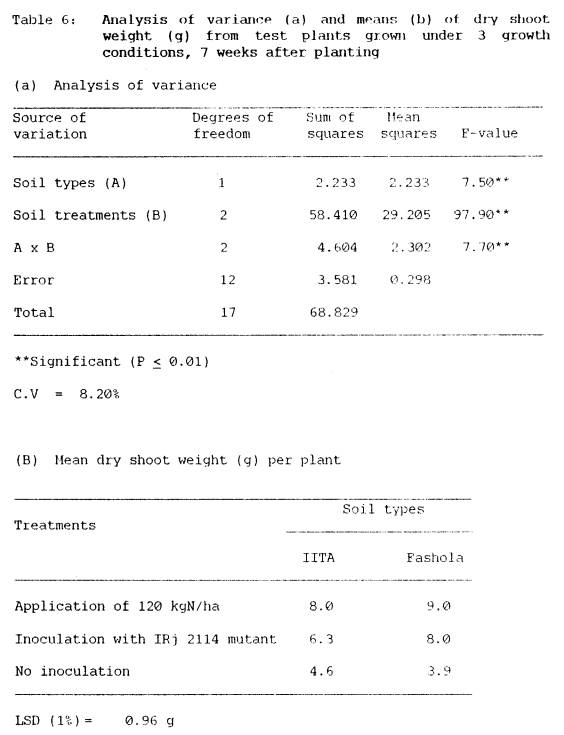
Dry
matter yields were significantly (P < 0.01) influenced by soil type,
seed inoculation and by their interaction effects (Table 6a),
In IITA soil, plants that
received fertilizer nitrogen had significantly higher dry matter yields than
those inoculated with IRj 2114 mutant.
This was not the case in Fashola soil, where dry matter yield of inoculated
plants was comparable to that of N-fertilized plants (Table 6b). Data presented, further showed that in both
IITA and Fashola soils, inoculated plants produced more dry matter (6.3 g/plant
IITA soil; 8.0 g/plant Fashola), than the uninoculated plants (4.6 g/plant IITA
soil; 3.9 g/plant Fashola soil).
Nitrogen contents in
shoots followed the pattern of city matter yields. However, soil type did not significantly influence the N-content
of plants (Table 7a). Highest nitrogen
content of shoots was recorded for plants inoculated with mutant IRj 2114 and
grown in Fashola soil (228 mgN/plant) although the amount was not significantly
different from those of nitrogen fed plants (Table 7b).
Results of plant growth
and nitrogen accumulation in shoots showed therefore that IRj 2114 mutant
rhizobium had a high level of activity.
4.4 DISCUSSION
Results obtained showed
that mutant IRj 2114 rhizobium nodulated effectively with soybean and also
contributed significantly to the nitrogen economy of the plant.
Nodulation of soybean in
Fashola soil, that had no native soybean rhizobia, was higher than in IITA soil
that had indigenous rhizobia (Table 4b).
However, inoculation also led to a near doubling of the number of nodules on soybean grown
in IITA soil. Inoculation of leguminous crops grown in soils free of specific
rhizobia causes good nodulation provided favorable conditions prevail
(Graham and Harris, 1982; Chowdhurry 1975).
This perhaps explains the high nodulation obtained in Fashola soil. The good response of soybean to inoculation
also obtained in IITA soil was attributable to the good nodulating qualities
and high competitive ability of the mutant IRj 2114 strain used.
Results obtained also
showed that the many nodules formed by IRj 2114 mutant rhizobium were small and
of low weights (Table 5b). This
observation was similar to that obtained by Rosendhal (1984). Singleton and Stockinger (1983) consider
that for rhizobia strains that form small nodules, compensation occurs by
increased nodulation. This ensures high
active nodule mass and a high capacity of the rhizobia to fix nitrogen.
Observations on crop
performance showed that inoculated plants developed vigorously, and their shoot
dry matter yields were comparable to those that received fertilizer nitrogen (Table
6b) and showed a level of about 84% symbiotic effectiveness (Gibson,
1987). Shoot dry matter weights are,
however, usually insensitive measures of the development of symbiosis
(Brockwell et al., 1985) and instead symbiotic, effectiveness was
used. Based on the levels of nitrogen
in plant shoots, it was evident that the symbiotic effectiveness was high when
IRj 2114 mutant was used as an inoculum for soybean. For plants grown in Fashola soil, the amount of nitrogen in
shoots attributable to biological nitrogen fixation was 158 mgN/plant. This was 69% of the total plant nitrogen
(Table 7b) which is equivalent to at least 227 kgN/ha fixed per hectare, the
minimum calculated by Neves et al. (1985) Patterson and Larue
(1983).
Results of this study
show clearly that IRj 2114 mutant rhizobium was very active in terms of
nodulation and nitrogen fixation and was therefore an effective microsymbiont
of soybean.
CHAPTER 5
EFFECT OF SOYBEAN-MAIZE CROPPING
SEQUENCES ON THE
INFECTIVENESS OF
INTRODUCED B. JAPONICUM AND
ON POPULATION OF SOYBEAN
RHIZOBIA
5.1 INTRODUCTION
There is evidence that
rhizobia grow in the soil in the absence of their host (Pena-Cabriales and
Alexander, 1983; Tuzimura and Watanabe, 1962; Rovira, 1961). However, the establishment of introduced
rhizobial population in soil has always been difficult; more so in soils under
crop rotations when the rhizobia have to live saprophytically (Hiltbold et
al., 1985).
In fields where legumes
such as soybeans have been frequently grown, populations of bradyrhizobia are
usually in excess of 1x104/g soil (Weaver et al.,
1972; Crozat et al., 1982; Mahler and Wollum, 1982). If such high rhizobial population were of
the inoculum strain, and was maintained following a non-legume crop in a
rotation, it would act as a source of inoculum for subsequent legume crops
(Brockwell et al., 1984; Brockwell et al., 1985),
and so eliminate the need for seed inoculation.
Little is known about the
effects of soybean/cereal cropping rotation on the establishment and infectiveness
of inoculum rhizobial strains. The
absence of such information hinders the utilization of biological nitrogen
fixation in crop production. In this
study, therefore, the effects of soybean/maize cropping sequences on population
of IRj 2114 inoculum strain of soybean rhizobia were assessed in the field and
in the glasshouse.
5.2 MATERIALS AND METHODS
5.2.1 Effect of cropping
sequences on soybean nodulation and on population of soybean rhizobia in the
field
In this study, assessment
was made of nodulation of soybean by the indigenous and introduced IRj 2114
mutant rhizobial strain, and of populations of soybean rhizobia in the soil,
for four soybean/maize cropping sequences over three seasons.
5.2.1.1 Establishment of field experiments
The experiments commenced
in June 1984. In the first season (June
- September, 1984), population of mutant IRj 2114 was established in the field
by planting soybean seeds inoculated with the mutant Rhizobium. Three plots, each measuring 27m by 9m, were
used for planting.
Based on studies reported
elsewhere (Abd-el Ghafter, 1976; Sayed, 1979), seven day old broth culture of
the mutant rhizobia diluted ten times was used to inoculate soybean seeds
before planting. 100 ml of the
diluent were mixed with 15.0g of peat and 4.0ml of gum arabic solution (as an
adhesive). The resulting slurry was
then used to inoculate 100.0g of soybean seeds. Analysis, using the most probable number (MPN) method
(Sub-Section 5.2.1.3.2) showed that the rate of inoculation was approximately
108 rhizobia per seed.
Inoculated soybean seeds
were planted in the plots, on 13th June 1984, at a spacing of 75 by 10 cm. Two seeds were planted her hole and, 14 days
after
planting, seedlings were thinned to one plant per hill. This gave a population of about 3.24 x 105
soybean plants per hectare.
In the second season
(October 1984 - January 1985), each of the three plots was divided into two
sub-plots, each measuring 13m by 9m. After random assignment, one sub-plot was
planted with soybean and the other with maize.
Planting was done on 4th October, 1984.
Uninoculated soybean (or maize) seeds were used for planting, and the
crop established as described above.
Maize was planted at a spacing of 75 cm by 25 cm, and a rate of two
seeds per hole. It was later thinned to
one plant per hill, 2 weeks after planting, thus giving a maize population of
about 5.3 x 104 plants per hectare.
In the third season
(February - May 1985), each sub-plot was again sub-divided into two equal portions measuring 6m
by 9m. Maize and soybean were then
assigned randomly to each portion, uninoculated seeds planted on 13th February
1985 and the crops established as described above.
The four different
cropping sequences obtained from the three plantings were as follows:
(i) soybean - soybean - soybean (SSS),
(ii) soybean - soybean -
maize (SSM),
(iii) soybean - maize –
soybean (SMS),
(iv) soybean - maize – maize (SMM).
The development of the
cropping sequences in the three is illustrated by Figure 1.
In all seasons, crops were
kept free of weeds by regular weeding using hand hoes. The crops received uniform application of
potassium (60kg K2O/ha), phosphorus (100kg P2O5/ha)
and molybdenum (1kg Mo/ha) fertilizers as described in Chapter 3, Section 3.2.
During dry spells, crops
were irrigated using over-head sprinklers.
Leaf pests on soybeans were controlled with nuvacron (dichlorovos),
applied, only when necessary, using an electrodyn sprayer that minimized
insecticide drift (Singh, 1981). This,
and the fact that nuvacron is rapidly decomposed by the plant, minimized any
possible effects of pesticide residues on soil rhizobia.
At the end of each
season, mature crops were cut at ground-level using cutlasses. Crop trash was raked off the plots and soil
samples then taken before preparing the land for the next planting.
5.2.1.2 Assessment of soybean nodulation
Each season, sampling was
carried out 49 days after crop planting and assessment made of (i) total
nodulation per soybean plant and (ii) percentage modulation due to the
introduced IRj 2114 mutant rhizobia.
Twenty soybean plants were randomly selected from middle rows of each
plot, sub-plot or sub-subplot, and carefully uprooted using a hand shovel. For each plant, nodules on the roots and
those that dropped off the roots at harvest were collected, washed and
counted. The nodules were stored in a
refrigerator for a maximum period of 14 days before completion of typing for
antibiotic resistance to assess the level of soybean nodulation due to
introduced mutant rhizobium.
Nodule typing was carried
out using the procedure described by Obaton (1973). Thirty nodules were randomly selected for each cropping sequence
and singly surface sterilized using mercuric chloride (Vincent, 1970). Each nodule was gently squeezed between the
tips of ethanol-flamed forceps and immersed into 1 ml of sterile yeast mannitol
broth (YMB). Inoculated YMB was
thoroughly mixed, after which a sterile loop was, used to aseptically transfer
samples from the YHB to plates containing either plain yeast mannitol agar
(YMA), or YMA to which was added streptomycin at 1,000 μg/ml of YMA.
One column of grids of
each plate (6 squares) was allocated to IRj 2114 mutant culture as a
check. Thirty of the remaining grids
were then typed with nodule samples from the inoculated YMB. The typing was replicated three times.
Plates were incubated at
28˚C for seven days and the rhizobial growth scored. Percentage nodulation caused by the IRj 2114
mutant Rhizobium was calculated using the formula:
X=
y/m X 100
where X = percent
nodulation due to mutant rhizobium,
y = positive scores on
plate with streptomycin,
and m = positive scores on plate with plain YMA.
5.2.1.3
Enumeration of soil rhizobia using the "Most Probable Number"
(MPN) technique
When enumerating rhizobia
in the soil or on seeds, the "most probable number" (MPN) technique
(Tuzimura and Watanabe, 1961) as modified by Weaver and Fredrick (1982) was
used. The technique is based on plant
infection and determines the number of viable rhizobia in the presence of other
organisms. Sterile plastic plant growth
pouches, obtained from Scientific Products, Evanston, Illinois, (U.S.A.), were
used. Rhizobial counts were made for soil
samples collected. The test was
conducted in a small screen-house.
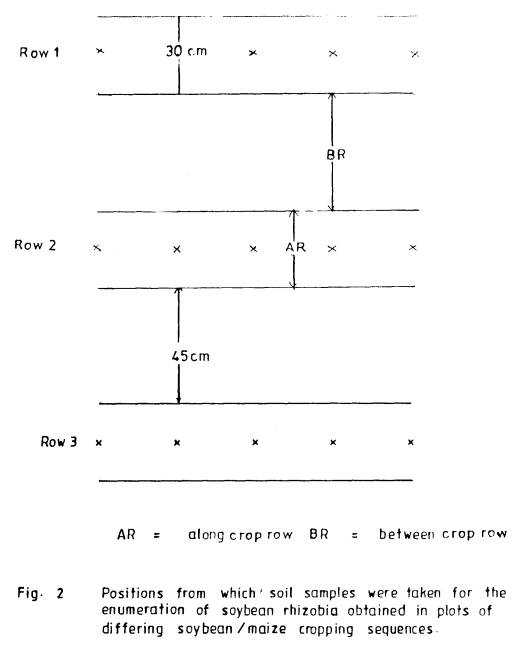
5.2.1.3.1 Sampling of field soil
Assessment of populations
of soybean rhizobia in the different experimental plots was carried out by
sampling soil at the end of each cropping season. After clearing crop residues from the plots, soil samples, taken
at a depth of 0-15 cm, were randomly obtained from two sampling position (i)
along the crop rows (AR) and (ii) between the rows (BR) as shown in Figure
2.
For each sampling
position, 20 samples were collected, using a soil auger of 6.0 cm diameter, and
a composite sample made for each plot.
Sub-samples taken from these composite samples were then used to
enumerate soybean rhizobia using the MPN technique.
5.2.1.3.2 Preparation of growth pouches and
establishment of test plants
Twenty pouches per soil
sample were used for the test. Each
pouch was divided into two equal compartments using a plastic heat sealer.
Paper wick for each pouch was also cut into two and inserted into each pouch
compartment. This procedure ensured
that the few available pouches were enough for the test, and the pouches
occupied limited space in the screenhouse.
Pouches were packed in handling racks and 20 ml of nitrogen-free plant
nutrient solution (Appendix 3) added to each compartment. The openings of pouches were wrapped with a
sheet of aluminum foil and the pouches then sterilized by autoclaving at 120°C
for 30 minutes. They were left to cool
to room temperature before use.
Sorted soybean seeds used
in the test were surface sterilized with mercuric chloride (Vincent, 1970) and
pre-germinated by incubating in sterile moist cotton wool in petri-dishes at 28°C
for 3-5 days. Seedlings with clean
radicles and free of fungal growth were selected and planted in pouches under
aseptic conditions. Two seedlings were
established in each pouch compartment.
The pouches were kept in the screen-house for one week after which well
established seedlings were inoculated with rhizobial suspensions being
tested.
5.2.1.3.3 Inoculation of
plants and enumeration of rhizobia
Seedlings in each pouch
compartment were inoculated using 1 ml of soil rhizobial suspensions. The suspensions were obtained by shaking
10.0g soil samples in 90 ml of sterilized tap water. The resulting suspension was of 10-1 dilution. Using 10 ml of this suspension, a 10-fold
dilution of the suspension was made with the resultant dilution levels ranging
from 10-1 to 10-10.
Plants in four replicate
pouch compartments were used per dilution.
Apart from these pouches, negative and positive control pouches were
also established. Negative control
pouches contained plants not inoculated with soil or rhizobia suspensions;
these indicated whether cross contamination occurred during growth. Positive
control plants were inoculated with mutant rhizobium, and they helped to
monitor the suitability of plant growth conditions for nodulation.
Pouches were supported in
groups of 60 in wire racks, each rack contained test pouches and the positive
and negative controls. The racks were
placed in a screen-house maintained at 28˚C, with filtered air flow. About 1.7 x 104 lux of light
intensity was provided from over-head fluorescent bulbs. Throughout the study period, 20 ml of
sterile dilute nitrogen-free nutrient solution and distilled water were added
in each pouch compartment on alternate days.
Although positive controls showed nodulation within three weeks, test
plants were kept for four weeks to ensure that adequate time was given for
nodulation.
At the end of four weeks,
roots of all plants were examined for nodulation. All replicate compartments with one or more nodules on the plants
were scored as positive. For each
dilution level, the total number of positive replicate compartments were
obtained. A grand total of the positive
scores was obtained for each soil sample tested by adding total positive scores
for the 10 dilution series. From the
Most Probable Number table (Appendix 4), the most likely number of rhizobia
corresponding to a particular number of positive scores was obtained for the
least dilute number of the series. The
estimated number of rhizobia occurring per gram of soil was calculated, in
accordance with procedure given by Vincent (1970), using the formula:
(m x d)
X = ------- x 100
(v x g)
Where X = number of
rhizobia per gram of soil,
m = most likely number
from the MPN table for the lowest
dilution of the series,
d = lowest dilution,
v = volume of aliquot
applied to plants,
and g = weight of soil
sample.
The most probable numbers
(MPN) of soybean rhizobia per gram of soils were transformed to log10
MPN or log10 (MPN + 1), to ensure detection of differences in
populations of the rhizobia (Russek and Caldwell, 1983; Crozat et al.,
1982). Data collected were then
subjected to analysis of variance (ANOVA) and means for the first and second
seasons compared using least significant difference (LSD). Single degree of freedom comparisons in the three
orthogonal sets (SSS vs SSM, SMS vs SMM and SSM vs SMS and SMM) and their
interactions with two sampling positions along (AR) and between (BR) the crop
rows were made on means obtained in the third season.
5.2.2 Glasshouse
evaluation of the establishment of soybean
rhizobia in fallow soil and in soils planted with soybean
or maize in the soils
The aim of this study was
to assess, under controlled conditions, the survival, colonization and
establishment of, both the introduced and indigenous soybean rhizobia in the
presence of soybean host and non-host rotational maize and in fallow soil. The experiment was set up in a glasshouse
during March 1985 using IITA soil.
Nine medium sized pots
(top diameter 20 cm, depth 18 cm) were each filled with 3 kg of soil as
described in Chapter 4 (Section 4.2).
Soils in the pots were wetted with distilled water before inoculation
with IRj 2114 mutant rhizobium.
Ninety millilitres of seven
day old culture of mutant rhizobium were put to dilution of 10-1.
Soil in each pot was inoculated
with the diluent at a rate of 100 ml per pot, poured on the surface of the
soil. The inoculum was then thoroughly
mixed into the soil using clean hand shovels.
Enumeration of soybean rhizobia from soils based on composite samples
showed that inoculation resulted in approximate populations of 108
rhizobia per gram of soil.
After soil inoculation,
three sets of pots were planted with maize, soybean or left fallow [without any
plant growth, Jensen and Sorensen (1987)].
Five seeds of each crop were planted per pot and the seedlings thinned to three per pot,
two weeks after planting. Because soybean nodules are known to start decaying
and releasing rhizobia into the soil after 70 days from planting (Bushby,
1981), the soils were sampled 70 days after crop planting. A second crop of soybean and maize was then
established in the same pots as described above, but without re-inoculated with
the rhizobium. The double planting
ensured that meaningful data were obtained that covered the 120 days of crop
development in the field.
The replicate pots were
arranged on a bench in a randomized complete block design. They were separated from each other by 20 cm
wide space. For both plantings, all
pots were watered regularly using tap water.
Composite nitrogen-free nutrient solution (Appendix 3) was also added at
the rate of 20 ml per pot every week.
However, phosphorus was not reapplied for the second crop since the single
superphosphate provided residual phosphorus in adequate amounts.
5.2.2.1 Enumeration of soil rhizobia using the MPN
technique
Sixty days after
planting, maize and soybean shoots were trimmed off. To allow for the roots and nodules to decay, soils in the pots
were regularly mixed and watered for a further ten days before soil sampling
was done. After the interval, 10.0g
composite samples of soils were obtained for each treatment combination and
used in the enumeration of soybean rhizobia.
Most probable numbers
(MPN) of soybean rhizobia per gram of soil were transformed using log10,
and the data subjected to analysis of variance (ANOVA). The means compared using the least
significant difference (LSD) test at 1% level.
5.3 RESULTS
5.3.1 Nodulation
of soybean under different soybean-maize cropping
sequences in field
Data on soybean
nodulation under continuous soybean cropping (SSS) are presented in Table
8. Both the total number of nodules per
plant and percentage of nodules formed by mutant rhizobium varied significantly
(P < 0.05) with the cropping season (Table 8a).
Nodulation increased with
cropping seasons (Table 8). In the
first season, the average number of nodules per plant was 29. This increased significantly in the second
season to an average of 39 nodules per plant, an increase of 34%. Further increase occurred in the third
season, however, it was only 15% over the level obtained in the second season
and the increase was not significant.
Nodule occupancy by IRj
2114 mutant rhizobium also increased with the cropping seasons (Table 8b). Fifteen percent of nodules of the first
season crop were due to the mutant rhizobium.
During the second and third seasons, nodule occupancy by the introduced
rhizobium increased approximately 2.5 and 4.0 times respectively over the level
obtained during the first season.
When soybean was grown in
rotation with maize (SMS), the maize grown during second season adversely
affected nodulation of subsequent soybean crop (Table 9). Analyses of variance of data collected
showed that types of cropping sequences significantly (P < 0.01)
influenced nodulation of soybean in the third season (Table 9a).
Mean number of nodules
per plant ranged from 45 for continuous soybean (SSS) to 26 for the
soybean-maize rotation (SMS) (Table 9b). Although the percentages of nodules
due to the mutant rhizobium obtained under the SSS and SMS cropping sequences
were not significantly different (Table 9a), lower recovery of nodules of the
introduced rhizobium was obtained under soybean-maize rotation (SMS) (42%) as
compared to continuous soybean cropping (SSS) (60%). Generally, soybean-maize rotation (SMS) did not favor
infectiveness of soybean rhizobia.
5.3.2 Population
of soybean rhizobia under different soybean-maize
cropping sequences in
field
Observations on the
effects of four soybean-maize cropping sequences (SSS, SSM, SMS and SMM) on
field populations of soybean rhizobia at two sampling positions are presented
in Tables 10, 11 and 12.
Throughout the study,
cropping sequence significantly (P < 0.05 and 0.01) influenced
soybean rhizobial counts. Populations
of rhizobia also varied significantly (P < 0.05 and 0.01) along crop
rows (AR) and between crop rows (BR) (Tables 10a, 11a and 12a). There were
substantially larger numbers of rhizobia in the regions of crop growth, along
the rows, as compared to the inter-row space.
Cultivation of inoculated
soybean in the first season resulted in overall increase in rhizobial
population in the field, although population of rhizobia found between crop row
(BR) were lower than the original population of indigenous rhizobia (Table
10a). The density of rhizobia found
along crop rows (AR) averaged 9.3 x 103/g soil, while that obtained
between crop rows (BR) was 3.8 x 102/g soil. These populations were approximately 1600%
greater than, and 32% less than the original population of indigenous soybean
rhizobia (Appendix 1), respectively.
In the second season,
when the first season soybean crop was followed by either soybean or maize, significantly
(P < 0.01) higher population of soybean rhizobia was obtained under
continuous soybean cropping (SS) than for soybean-maize (SM) rotation at boar
sampling positions (Table 11b). Under
continuous soybean cropping (SS), rhizobia counts per gram of soil along the
crop rows and in the inter-row spaces were 1.6 x 106 and 6.3 x 104
respectively. These were approximately
1000 and 63 times greater than those obtained
after the maize crop
(SM). From the data, it was also
evident that maize depressed soybean rhizobial population. For instance, the density of rhizobia
obtained along the crop rows (AR) after maize crop (SM) was 1.6 x 103
rhizobia/g soil which is 5.8 times lower than the level (9.3 x 103
rhizobia/g soil) obtained after the first season soybean crop.
Data on soybean rhizobial
count in the third season obtained after the SSS, SSM, SMS and SMM cropping
sequences are presented in Table 12.
Population of rhizobia varied significantly (P < 0.01) with
cropping sequence and sampling position, and was also significantly influenced
by the type of crop planted in the second season (Table 12a).
Soybean rhizobial counts
along crop rows (AR) of the third season crops (Table 12b and Figure 3) showed
that significantly (P < 0.01) higher populations of rhizobia occurred
in soils when both soybean and maize followed two consecutive soybean crops
(SS) than when maize was grown as the second crop. There was no significant difference in soybean rhizobial counts
between rows (BR) under SSS, SSM and SMS cropping sequences, but significantly
(P < 0.05) soybean rhizobial populations were obtained under SMM
(Figure 3).
5.3.3 Population
of soybean rhizobia in fallow soil, soybean and
maize cropping in the
glasshouse
The establishment and
survival of soybean rhizobia in potted IITA soil left under fallow or cropped
to soybean or maize are summarized in Table 13 and Figure 4. Rhizobial populations varied significantly
(P < 0.01) amongst the different treatment pots (Table 13a). There was also substantial variation in the
patterns of rhizobial populations with time as indicated by the significant (P <
0.05) interaction between
cropping treatment and time of sampling (Table 13a).
During the first 70 days
of planting, the rhizobial numbers per gram of soil declined from 1.00 x 108,
an initial level obtained after inoculation to 1.58 x 102, 6.31 x 102
and 2.51 x 102 for fallow soil, soybean and maize cropping
respectively. These values were not
significantly different (Table 13b). In
the subsequent 70 day period of planting, population of soybean rhizobia in
soil cropped with maize declined further while those in fallow soil and in soil
cropped with soybean increased (Figure 4).
At 140 days after soil inoculation, rhizobial counts per gram of soil in
pots planted with maize (50) was significantly (P < 0.05) lower than
those obtained for soybean (2512) or fallow (630) pots (Table 13b).
The results showed that
maize suppressed rhizobial multiplication, and this agrees with observations
obtained in the fiel.
5.4 DISCUSSION
Observations made in this
study showed that nodulation of soybean and population of soybean rhizobia in
soil were greatly influenced by soybean-maize cropping sequences. There was a low nodule recovery for the introduced
soybean rhizobium at the end of the first season. In the two subsequent seasons, continuous soybean (SSS)
encouraged high infectivity of mutant IRj 2114 rhizobium, and the total
rhizobia population in the soil. Under
rotation with maize (SMS), however, total soybean nodulation, percentage
recovery of nodules due to the inoculant rhizobium, and rhizobial
population
in the soil were all
adversely affected.
Increased total
nodulation of soybean plants, and nodulation due to the introduced IRj 2114
mutant rhizobium obtained and continuous soybean cropping (Table 8) reflected
the important role that host plants play in the establishment and
multiplication of rhizobia in the field.
Through nodule disintegration (Bushby 1981; Brockwell et al.,
1988) and multiplication in the rhizosphere (Rovira, 1961), population of the
introduced IRj 2114 mutant increased as indicated by higher nodule percent
recoveries obtained in the second (38%) and third (60%) seasons. Results obtained in the present study are in
conformity with observations made by Kolling et al.,
(cited by Freire, 1976) and Dunigan et
al. (1984).
The results also showed
adversely affected nodulation of soybean plants. Total nodulation of the plants and recovery of nodules formed by the
mutant rhizobium were substantially lowered (by 42% and 18% respectively) for
the third season soybean crop that followed maize (Table 9). This adverse effect of maize crop was also
reflected by the significantly (P < 0.01) lower soybean rhizobial
populations obtained when maize was grown as a second crop (Table 12). These field observations on the populations
were supported by data obtained from pot. experiment (Table 13 and Figure 4).
It is known that in the
absence of appropriate host crops, soybean rhizobial populations in the soil
decline. Hiltbold et al.
(1985), for instance observed a decline of soybean rhizobial population when
cotton followed soybean in rotation.
Nutman and Hearne (1979) also observed declining populations of rhizobia
under continuous cereal crops. Poor
survival and multiplication of rhizobia under the influence of non-host
rhizosphere was probably responsible for this decline in rhizobial populations.
Data collected also
showed that populations of soybean rhizobia were consistently higher along crop
rows (AR) than in the spaces between the rows (BR) (Tables 10, 11, 12). Johnstone (1964) and Moerman (1965) also
found, after soybean harvest, that while there were virtually no soybean
rhizobia between the crop rows, soil near the crowns of the old plants (about
15 cm radius) was still heavily infected.
It is reported that
rhizospheres of both host and non-host plants significantly influence the
growth of soil rhizobial populations (Rovira, 1961; Robinson, 1967;
Pena-Cabriales and Alexander, 1983; Jensen and Sorensen, 1987). Diatloff (1969), for example, found a high
stimulation of soybean rhizobia by both oats and soybean. Chowdhurry et al.
(1968) also demonstrated the stimulating
effects of rhizosphere in the sterile soil and showed that serradella rhizosphere
markedly stimulated Rhizobium lupini.
It is generally
recognized that rhizobia grow and multiply in rhizospheres in response to
available nutrient (carbohydrates, amino acids and vitamins) contained in root
exudates (Date and Brockwell, 1978). The
high density of roots found along crop rows (AR) and rhizobia release into soil
by decaying nodules were therefore responsible for the high populations of
rhizobia found in this region as compared to the inter-row spaces.
It was evident from the
study that successful establishment and build-up of populations of rhizobia was
dependent on the presence of host plant.
It was also evident that in soybean-maize rotation, successful
establishment and rapid multiplication of an introduced soybean rhizobium depends
significantly on whether soybean or maize follows the first inoculated soybean
crop.
CHAPTER 6
GENERAL DISCUSSION AND CONCLUSIONS
The work reported in this
thesis investigated the possibility of obtaining long term benefits from
introduced highly effective strains of soybean rhizobia (Bradyrhizobium japonicum) in the existing cropping
systems, particularly rotations.
Soybean and other legumes
are normally grown in rotation with cereals and other crops to reduce the
build-up of diseases, pests and weeds, and also to exploit the contributions of
the legumes, through nitrogen fixation, to the soil nitrogen economy. In such a cropping system, an introduced B.
japonicum strain has to compete with the existing microflora for
available substrate for growth. It has
to survive in the absence of specific host and later compete for nodule sites
whenever the specific host is planted (Freire, 1976; Elkan, 1987). There is a need therefore to establish a
better understanding of the persistence of both introduced and indigenous
soybean rhizobial populations in soils under different cropping sequences.
Marked positive responses
of soybean to inoculation with IRj 2114 mutant rhizobium obtained in this study
can be attributed to the high competitiveness of the strain and advantages
provided by the inoculation process. By
seed inoculation, high numbers of selected rhizobia are concentrated in the
proximity of emerging plant roots thus
creating high chances of
infection for the strain. For an
introduced strain, successful establishment, multiplication and effectiveness
will depend however, on its ability to adopt to its new environment (Parker et al.,
1977; Vincent, 1977; Dunigan et al., 1984).
During the present study,
soybean nodulation in the first season (after inoculation) due to the mutant
rhizobium was very low (15%) and only buildup after repeated soybean cropping
to an average of 51% in third season (60% for SSS and 42% for SMS). Low initial percentage recovery of nodules
formed by introduced rhizobial strains have also been reported by earlier
workers (Johnson et al., 1965; Caldwell and Vest, 1970; Cardwell
and Johnson, 1971). In Kawanda, Uganda,
for example, response to inoculation of soybean was obtained from subsequent
soybean crops rather than from the inoculated crop (Anon., 1953). It seems, therefore, that an improved
soybean rhizobial strain introduced through inoculation undergoes intense
selection for survival and competitiveness during the first cropping
season. Successful adaptation is followed
by the build-up of populations of the introduced rhizobium in the soil.
Because of this apparent
period of adaptation, following introduction, the type of crop that follows the
inoculated legume crop is important for the successful establishment of introduced
rhizobium. In the present study, for
instance, there was low total nodulation of soybean plants and percentage of
nodules formed by the introduced mutant rhizobium, in the third season, when
maize followed the inoculated soybean crop.
The maize crop grown in the second season did not stimulate
multiplication of the introduced rhizobium.
Although non-legume
rhizospheres are known to stimulate rhizobial growth, their effects are
generally smaller than stimulation caused by the legume rhizospheres (Date and
Brockwell, 1978, Pena-Cabriales and Alexander 1983). This low stimulation effects of non-legume crops means therefore
that careful consideration should be given to the type of cropping sequences to
he followed when introducing new highly effective strains of soybean and other annual legume
rhizobia. The present study showed for instance that soybean inoculated
with introduced rhizobium should be followed by a second soybean crop to ensure
adequate establishment and build-up of introduced rhizobium.
Finally, it is suggested
that studies he carried out to establish the effects of soybean rotation with
crops other than maize like some legumes on infectivity and effectiveness of
introduced rhizobial strains. To gain a
better understanding of the relationships between nodulation ability,
competitiveness and saprophytic competence of introduced strains, the
immunoflourescence technique could be used to study directly the autoecology of
the rhizobia in soils and in natural rhizospheres (Bohlool and Schmidt, 1973;
Reyes and Schmidt, 1979, 1981). This
would be a significant improvement over the MPN and antibiotic techniques used
in this study.
REFERENCES
ABD-EL-GHAFFAR,
S.A.M. (1976). "Some Factors Affecting Nodulation and
Nitrogen Fixation of Soybean (Glycine max).” M.Sc. Thesis. Fac. Agric. Univ. of
Alexandria, Egypt.
ALEXANDER, M. (1977).
Ecology of Nitrogen-fixing organisms, In: A.A. Ayanaba and P.J. Dart
(eds.) "Biological nitrogen Fixation in Farming Systems". Wiley, New York, pp. 100-119.
ANON. (1982).
Course Manual for Training Course on the Application of Rhizobium
technology in commercial legume growing at the University of Nairobi, Kenya;
held from 4 June to 2 July, 1982, Nairobi.
ANON. (1969).
Annual Report of the Agricultural Research Council of Malawi 1969,
Microbiology. pp. 18-20.
ANON. (1953).
Notes on the Principal Annual crops.
Department of Agriculture, Uganda Protectorate. pp. 31-32.
ASHLEY, J. (1973).
The status of soybean in Uganda.
In: "Uganda Agriculture for Better Living." Proc. of the 1st Symposium of the Uganda
Soc. of Agron. pp. 56-64.
AUCKLAND, A.K. (1970).
Soybean improvement in East Africa.
In: "Crop Improvement in East Africa" (C.L.A. Leakey ed.). pp. 129-159.
AYANABA, A.; AHMAD, M.H.;
EAGLESHM, A.R.J.; HAUSSOUNS, S.; SEAMAN,B., MULONGOY, K. and PULVER, E.L.
(1981). Examining the potential for
inoculant use with cowpeas in West African Soils. Trop. Agric. Trinidad 58(4): 325-335.
BELL, F. and NUTMAN,
P.S. (1971). Experiments on nitrogen fixation by nodulated lucerne. In: T.A. Lie and E.C. Mulder (eds.).
"Biological Nitrogen Fixation in Natural and Agricultural
Habitants." Plant and Soil Spec.
Vol. pp. 231-264.
BERGENSEN, F.J. (1977).
Factors controlling Nitrogen Fixation by Rhizobia. In: A.A. Ayanaba and Dart., P.J. (eds.)
"Biological Nitrogen Fixation in farming systems of the tropics." pp. 153-165.
BOHLOOL, B.B. and
SCHMIDT, E.L. (1973). Persistence and competition aspects of Rhizobium
japonicum observed in soil by immunoflourescence microscopy. Soil Soc. Am. Proc. 37: 561-564.
BREMNER, J.M. (1960).
Determination of nitrogen in soil by Kjeldahl method. J. Agric. Sci. 55. 1-23.
BROCKWELL, J., GAULT,
R.R.; CHASE, D.L.; TURNER, G.L. and BERGERSON, F.J. (1985). Establishment and
expression of soybean symbiosis in a soil previously free of Rhizobium japonicum. Austr. J. of Agric. Research. 36(3):
397-409.
BROCKWELL, J., HERRIDGE,
D.F., MORTHORPE, L.J. and ROUGHLEY, R.J. (1988). Numeric effects of Rhizobium population on legume symbiosis. In: Beck, D.P. and Materon, L.A. (Eds). "Nitrogen Fixation by Legumes in
Mediterranean Agriculture."
Martinus Nijhoff, Dordrecht, The Netherlands. pp. 179-193,
BROCKWELL, J., ROUGHLEY,
R.J. and HERRIDGE, D.F. (1984). Impact of rhizobia established in a high
nitrate soil on a soybean inoculant of the same strain. In: "Advances in Nitrogen Fixation
Research". Proc. 5th Int. Symp.
Nitrogen fixation. Veeger, C. and W.E.
Newton (eds.). pp. 328.
BURNS, R.C. and HARDY,
R.W.F. (1975). Nitrogen fixation in bacteria and higher
plants. Springer Verlag, Berlin,
Heidelberg, N.Y. p. 189.
BURTON, J.C. (1972).
Alfalfa Science and Technology.
Edited by Clarence, H.H. Am. Soc. Agron. No. 15: Madison Wisconsin, U.S.A.
BUSHBY, H.V.A. (1981).
Current perspectives in nitrogen fixation. In: Gibson, A.H. and William,
E.N. (eds.). Aust. Acad. of Sci. p.
432.
CALDWELL, V.B. (1982).
Sources of yield increase. Agron. J. 774: 984-990.
CARDWELL, B.E. and GRANT,
V. (1970). Effect of Rhizobium japonicum on soybean
yields. Crop Sci. 10: pp. 19-21.
CASSMAN, K. (1979).
"The P Nutrition of Two Grain Legumes Affected by Mode of
Nutrition." Ph.D. Thesis,
University of Hawaii.
CHAPMAN, A.L. and MYERS,
R.J.K. (1987). Nitrogen contributed by grain legumes to
rice grown in rotation on the Cununurra soils of the Orda irrigation area,
Western Australia. Aust. J. Exp. Agric.
27: 155-163.
CHATT, J. (1981).
Towards new catalysts for nitrogen fixation. In A.H. Gibson and W.E. Newton (eds.). "Current Perspectives in Nitrogen Fixation." Austr. Acad. Sci. pp. 15-21.
CHOWDHURY, M.S.; K.C.
MARSHALL and C.A. PARKER. (1968). Growth rates of Rhizobium trifolii
and Rhizobium lupini in sterilized soils. Aust. J. Agric. Res.,
19: 919-925.
CHOWDHURY, M.S. (1977).
Response of Soybean to Rhizobium inoculation at Morogoro, Tanzania. In: Ayanaba, A. and P.J. Dart (eds.)
"Biological Nitrogen Fixation in Farming Systems of the Tropics". pp.
245-253.
COCHRAN, W.G. (1950).
Estimation of bacterial densities by means of the "Most probable
number". Biometrics 6: 105-116.
CROZAT, Y.;
CLEYET-MARCL,; GIRAND, J.J. and OBATON, M.
(1982). Survival rates of Rhizobium japonicum populations
introduced in different soils. Soil
Biol. Biochem. 14: 401-405.
DANSO, S.K.A. and ALEXANDER,
M. (1975). Regulation of predation by prey density: the protozoan - Rhizobium
relationship. Appl. Microbiol. 29:
515-521.
DATE, R.A. and BROCKWELL,
J. (1978). Rhizobium strain competition and host interaction for
nodulation. In: Wilson, J.R. "Plant
Relations in Pastures." CSIRO,
East Melbourne. pp. 202-216.
DATE, A.R., and HALLIDAY,
J. (1987). Collection, Isolation, Cultivation and Maintenance of
rhizobia. In: Elkan, H.G. (ed.)
"Symbiotic Nitrogen Fixation Technology" Marcel Dekker, Inc. pp.
1-27.
DAZZO, F.B. (1980).
Infection processes in the Rhizobium-Legume Symbiosis. In: Summerfield, R.J. and Bunting, A.H.
(eds.). "Advances in Legume Science." Royal Botanic Gardens, Kew, N.K. pp. 49-59.
deSOUZA, D.T.A. (1969).
Legume nodulation and nitrogen fixation studies in Kenya. E. Africa Agric. For. J. 33(3): 299-305.
DIATLOFF, A. (1967).
Effect of nitrification of a black earth. Soil on Legume Nodulation. Old J. Agric. Anim. Sci. 24: 323-327.
DIATLOFF, A. (1969).
The introduction of Rhizobium japonicum to soil by seed
inoculation of non-host legumes and cereals.
Austr. J. Expt. Agric. Anim. Husb. 9: 357-360.
DUNBAR (1975). The Annual Crops of Uganda. Second Edition. East African Literature Bureau. pp. 66.
DUNIGAN, E.P., BOLLICH,
P.K., HATCHINSON, R.L., HICKS, P.M., ZAUMBRECHER, F.C., SCOTT, S.G. and MOWERS,
R.P. (1984). Introduction and survival
of an inoculant strain of Rhizobium japonicum in soil. Agron. J, 76: 463-466.
ELKAN, G.H. (1987).
"Symbiotic Nitrogen Fixation Technology". Marcel Dekker, Inc.,
New York.
FAO (FOOD AGRICULTURAL
ORGANISATION OF UNITED NATIONS) (1983). Production Year Book of 1983. FAO, Rome, Italy.
FOX, R.H. and W.P.
PIEKIELEK (1988). Fertilizer Nitrogen
equivalence of alfalfa, birds foot trefoil, and red clover for succeeding corn
crops. J. Prod. Agric. 1: 313-317.
FRED, E.B.; BALDWIN, I.L.
and McCOY, E. (1932). "Root Nodule Bacteria and Leguminous
Plants." University of Wisconsin,
Madison, Wisconsin. No. 5.
FREIRE, J.R.J. (1976).
Inoculation of soybeans. In:
"Proceedings of workshop on exploiting the Legume-Rhizobium
Symbiosis in Tropical Agriculture."
Hawaii. pp. 335-379.
GIBSON, A.H. (1987).
Evaluation of Nitrogen Fixation by Legumes in the Greenhouse and Growth
Chamber. In: Elkan, G.H. (ed.),
"Symbiotic Nitrogen Fixation Technology." Marcel Dekker, Inc. N.Y. and Basel, pp. 321-370.
GOMEZ, L.L.A. (1968).
Rotation and yields of maize: Effect of a rotation with soybeans or
lucerne on maize yield. Bibl. 4 ES.
Central, Nac. Invest. Agropec. Palmira, Colombia. Agriculture trop. 24(4): 204-220.
GRAHAM, P.H. and HARRIS,
C.S. (1982) (eds.). "Biological Nitrogen Technology for
Tropical Agriculture." CIAT CALI,
Colombia.
GRAHAM, P.H. and HUBBELL,
D.H. (1975). Soil-Plant Rhizobium interaction in tropical
agriculture. In. E. Bornemisza and A.
Alvarado (eds.), "Soil Management in Tropical America." N.C. State University Raleigh, N.C.
GRAHAM, P.H., and PARKER,
C.A. (1964). Diagnostic features in characterization of the root-nodule
bacteria of legumes. Plant. Soil, 20:
pp, 383-396.
HAGEDORN, C. (1979).
Relationship of antibiotic resistance to effectiveness in Rhizobium
trifolii populations. Soil Sci.
Soc. Am. J. 43: 921-925.
HAM, G.E.; CARDWELL, V.B.
and JOHNSON, H.W. (1971). Evaluation of R. japonicum
inoculants in soil containing naturalized populations of rhizobia. Agro. J. 63: 301-303.
HAMDI, Y.A., Abd.ELSAMEA,
M.E. and LOUTFI, M. (1973). Nodulation of soybean under field
conditions. Zeut. for Bakt. IIBd. 120:
574-578.
HANSON, R.G.; J.A.
STECKER and S.R. MALEDY (1988). Effect
of soybean rotation on the response of sorghum - fertilizer Nitrogen. J.
Production Agric. 1: 318-321.
HARDY, R.W.F. and
HAVELKA, U.D. (1975). Nitrogen fixation research: A key towards food. In: P.H. Abelson (ed.), "Food Politics,
Economics, Nutrition and Research." Amer. Assoc. Adv. Sci. pp. 178-188.
HERRIDGE, D.F., ROUGHLEY,
R.J. and BROCKWELL, J. (1984). Effect of rhizobia and soil nitrate on the
establishment and functioning of the soybean symbiosis in field. Aust. J.
Agric. Res. 35: 149-161.
HESTERMAN, O.B.;
RUSSELLE, M.P.; SHEAFFER, C.C. and HEICHEL, G.H. (1987). Nitrogen utilization from fertilizer and
legume residues in Legume-corn rotations.
Agron. J. 79: 726-731.
HILTBOLD, A.E.;
PATTERSON, R.M. and REED, R.B.
(1985). Soil populations of Rhizobium
japonicum in a Cotton-corn-soybean rotation. Soil Sci. Soc. Am. J. 49: 343-348.
HITCHER, E.R. (1930).
The isolation of a bacteriolytic principle from the root nodule of red clover. J. Bacteriol., 19: 191-201.
IITA (INTERNATIONAL,
INSTITUTE OF TROPICAL AGRICULTURE) (1972).
Annual Report of International Institute of Tropical Agriculture (IITA)
Ibadan, Nigeria.
IITA (INTERNATIONAL
INSTITUTE OF TROPICAL AGRICULTURE) (1979). "Selected methods for Soil and
Plant Analysis". Ibadan, IITA.
IITA Manual Series No.1.
IITA (INTERNATIONAL
INSTITUTE OF TROPICAL AGRICULTURE) (1982).
Annual Report of International Institute of Tropical Agriculture (IITA),
Ibadan, Nigeria.
IITA (INTERNATIONAL
INSTITUTE OF TROPICAL AGRICULTURE) (1978).
Annual Report of International Institute of Tropical Agriculture (IITA),
Ibadan, Nigeria.
JENSEN, E.S. and
SORENSEN, L.H. (1987). Survival of Rhizobium leguminosarum
in soil after addition as inoculant.
FEMS Microbiol. Ecol. 45: 221-226.
JENSEN, H.L. (1958).
The classification of the Rhizobia.
In: "Nutrition of the Legumes." E.G. Hallsworth (ed.). pp. 75-86.
JENSEN, H.L. (1942).
Nitrogen Fixation in the leguminous plants. The influence of reaction on the formation of Root Nodules in
Medicago and Trifolium. Proc. Linn.
Soc. N.S.W. 66: 207-220.
JETHMALANI, S.C.; TIWARI
K.L.; MOTIRAMANI D.P. (1969). Soybean fertilization and inoculation. Indian Fmg. 19(4): 17-18.
JOHNSON, H.; MEANS, V.M.
and WELBER, C.R. (1965). Competition for nodule sites between strains
of Rhizobium japonicum applied as inoculum and strains in
soil. Agron. J. 57: 179-185.
JOHNSTONE, O.M. (1964).
Soil microbiology. In:
Agricultural Research Council of Central Africa (Rhod. Zamb. Mal.). Annual Report. 1964. pp. 20-21.
JONES, G.D. and LUTZ,
J.A. (1971). Yield of wheat and soybeans, oil and protein content of soybean
as affected by fertility treatments and deep placement of limestone. Agron. J. 63: 931-934.
JORDAN, D.C. (1984).
Family III. Rhizobiaceae Conn. 1938-254. In: N.R. Krieg and J.G. Holt (eds.). "Bergey's Manual of Systematic
Bacteriology. 1." Williams and
Wilkins Co., Baltimore. pp. 234-255.
JORDAN, D.C. (1982).
Transfer of Rhizobium japonicum Buchanan 1980 to Bradyrhizobium
gen. nov., a genus of slow growing, root nodule bacteria from leguminous
plants. Int. J. of Syst. Bacteriol. 32: 136-139.
JOSEY, D.P.; BEYNON,
J.L.; JOHNSTON, A.W.B. and BERINGER, J.E.
(1979). Strain identification
Rhizobium using intrinsic antibiotic, resistance. J. Appl. Bacteriol. 46: 343-350.
KEYA, S.O. and ALEXANDER,
M. (1975). Regulation of parasitism by host density; the Bdellovibrio Rhizobium
interrelationship. Soil Biol. Biochem.,
1. 231-237.
KEYA, S.O. (1977).
Nodulation and nitrogen fixation in legumes in East Africa. In: A. Ayanaba and P.J. Dart (eds.). "Biological Nitrogen Fixation in
Farming Systems of the Tropics." pp. 233-243,
KEYA, S.O. (1974).
"Effect of Parasite Bdellovibrio and Predatory Protozoa on Survival
of Cowpea Rhizobium in Culture and Laboratory Soil." Ph.D. Thesis, Cornell University, Ithaca. New York
KLECZKOWSKA, J. (1957).
A study of the distribution and the effect of bacteriophage of root
nodule bacteria in the soil. Can. J. Microbiol.
3: 171-180.
KRIEG, N.R. and HOLT,
J.G. (eds.) (1984). "Bergey's
Manual of Systematic Bacteriology."
Vol. 1. Williams and Wilkins Baltimore U.S.A. pp. 964.
LAL, R.
1975. Role of mulching techniques in
tropical soil and water management.
Ibadan, IITA. IV, (IITA
Technical Bulletin No. 1).
LOCKHEAD, A.G. (1952).
Soil microbiology. Ann. Rev.
Microbiol. 6: 185-206.
LONERAGAN, J.F. and
BOWLING, E.J. (1958). The interaction of Calcium hydrogen ions in
the nodulation of Subterranean Clover. Aust. J. Agric. Res. 9: 464-472.
LOWENDORF, H.S. (1980).
Factors affecting survival of Rhizobium in soil. Adv. Micro. Ecol. 4: 87-124.
LYON, T.L. and BIZZELL,
J.A. (1934). A comparison of several legumes with respect to nitrogen
production. J. Amer. Soc. Agron. 26:
651-656.
MAHLER, R.L. and WOLLUM,
A.G. (1982). Seasonal fluctuation of Rhizobium japonicum under a
variety of field conditions in North Carolina.
Soil Sci. 134: 317-324.
MANIL, P. (1958).
The Legume-rhizobia Symbiosis.
In: Hallsworth, E.G. (ed.)
"Nutrition of the Legumes."
Butterworths Sci. Publ. pp. 124-133.
MASEFIELD, G.B. (1958).
Some factors affecting nodulation in the tropics. In: Hallsworth, E.G. (ed.) "Nutrition
of the Legumes." Butterworths Sci. Publ. pp. 202-211.
McNEIL, D.L. (1982).
Variation in agility of Rhizobium japonicum strains to nodulate soybeans and maintain
fixation in the presence of nitrate.
Appl. Environ. Microbiol. 44:
647-652.
MEIKLEJOHN, J. (1954).
Notes on nitrogen fixing bacteria from East African Soils.
MINISTRY OF AGRICULTURE -
TANZANIA (1978). Annual Report 1978.
MOERMAN, O.M. (1966).
Soil microbiology. In: Agricultural Research Council of Central Africa
(Rhod. Zamb. Mal.). Annual Report. 1965 p. 29.
MOORMAN, F.R., JUO, A.S.R.,
and LAL, R. (1975). "The Soils of IITA.” Tech. Bull. No. 3.
International Institute of Tropical Agriculture, Ibadan, Nigeria.
MUGHOGHO, K.S. (1985).
The role and performance of nitrogen fertilizers in tropical
Africa. Paper presented at Symposium on
the Management of Nitrogen and Phosphorus Fertilizers in Sub-Saharan Africa at
International Institute of Tropical Agriculture, (IITA) Ibadan, Nigeria.
MUNNS, D.N. (1978).
Soil acidity and inodulation.
In: C.S. Andrea and E.S. Kamprath (eds.), "Mineral Nutrition of
Legumes in Tropics and Sub-Tropical Soils." CSIRO, Melbourne, pp.
247-263.
MUNNS, D.N. (1977).
Mineral nutrition and the legume symbiosis. In: "A Treatise on
Dinitrogen-Fixation." Section IV.
Agronomy and Ecology. Hardy, R.W.F. and Gibson, A.H. (eds.). John Wiley and Sons, New York.
NEVES, M.C.P., DIDONET,
A.D., DUQUE, F.F. and DOBEREINER, J.
(1985). Rhizobium strains effects on nitrogen transport and
distribution in soybeans. J. Exp. Bot.
36: 1179-1192.
NEWCOMB, W.; D. SIPPELL
and R.L. PETERSON (1979). The early
morphogenesis of Glycine max and Pisum sativum root
nodules. Can. J. Bot. 57: 2603-2616.
NUTMAN, P.S. and HEARNE,
R. (1980). Persistence of nodule bacteria in soil under long-term cereal
cultivation. In: "Rothamsted
Experimental Station, Annual Report 1979, Part 2." pp. 17-90.
NUTMAN, P.S. (1965).
Symbiotic nitrogen fixation In: W.V. Batholomew and F.C. Clark (eds.).
"Soil Nitrogen." Amer. Soc. Agron. Mon. 10: Madison Wis. pp. 360-383.
OBATON, M. (1977).
Effectiveness, Saprophytic and Competitive ability: three properties of Rhizobium
essential for increasing the yield of inoculated legumes. In: A.A. Ayanaba and
P.J. Dart (eds.). "Biological Nitrogen Fixation in Farming Systems of the
Tropics." pp. 128-133.
OBATON, M. (1973).
The use of Spontaneous antibiotic resistant-mutants for ecological study
of Rhizobium. In: T. Roswell (ed.), "Modern Methods in the Study of
Microbiol. Ecology. Bull. Ecol. Res. Comm. 17: NFR, Stockholm, pp. 170-171.
OKIGBO, B.N. (1978).
Grain legumes in the agriculture of the tropics. In: S.R. Singh, J.A.
Taylor and H.F. Van Emden (eds.). "Insect Pests of Grain Legumes."
Academic Press, N.Y., p. 454.
OKIGBO, B.N. (1976).
Role of legume in smallholding of the humid tropics. In: J.M. Vincent,
A.S. Whitney and J. Bose (eds.). "Exploiting the Legume-Rhizobium
Symbiosis in Tropical Agriculture." Coll. Trop. Agric. Misc. Pbl. 145:
Dept. Agron. Soil Sci. Univ. Hawaii. Manoa, Hawaii. p. 465.
OLSEN, F.J. and MOE,
P.G. (1971). The effects of phosphate and lime on the establishment, productivity, nodulation
and Desmodium intortum, Medicago sativa and Stylosanthes gracilis. E. Afr. Agric. For. J. 37: 29-37.
OSA-AFIANA, L.O. and
ALEXANDER, M. (1983). Effect of moisture on the survival of Rhizobium
in Soil. Soil Sci. Am. J. 43: 435-439.
PARKER, C.A., TRINICK,
M.J. and CHATEL, D.L. (1977). Rhizobia as soil and rhizosphere
inhabitants. In: R. Hardy and A.H.
Gibson (eds.). "A Treatise On Dinitrogen Fixation", Chap. IV,
Agronomy and Ecology. Wiley, New York. pp. 311-352.
PATTERSON, T.G. and
LARUE, T.A. (1982). Nitrogen fixation by soybeans; seasonal and
cultivar effects and comparison of estimates.
Crop Science 23: 488-492.
PENA-CABRIALES, J.J. and
ALEXANDER, M. (1979). Survival of Rhizobium in soil
undergoing dry. Soil Sci. Soc. Amer. J.
43: 962-965.
PENA-CABRIALES, J.J. and
ALEXANDER, M. (1983). Growth of Rhizobium in unamended
soil. Soil Sci. Soc. Amer. J. 47: 81-84.
PERKASEN, B. (1977).
"Differential Sensitivity to Soil Acidity of Legume-Rhizobium
Symbiosis.” Ph.D Thesis. Univ. of W. Australia.
PIMENTEL, D. (1976).
Food, nitrogen, energy. In: W.E. Newton and C.J. Nyman (eds.).
"Proceedings of the First Symposium on nitrogen fixation." Wash.
State Univ. Press. pp. 656-673.
PULVER, E.L., BROCKMAN,
F. and WIEN, H.C. (1982). Nodulation of soybean cultivars with Rhizobium
spp. and their response to inoculation with R. japonicum. Crop Science.
22: 1065-1070.
PURCHASE, H.F. and
NUTMAN, P.S. (1957). Studies on the physiology, of nodule
formation. VI. The influence of
bacterial numbers in nodule initiation. Ann. Bot. Lond. 21: 439-354.
QIUSPEL, A. (1974).
"The Biology of Nitrogen Fixation." Pbl. North Holland Publ.
Co., Amer. Elsevier.
RACHIE, K.O. (1977).
The nutritional role of grain-legumes in the lowland humid tropics. In:
A.A. Ayanaba and P.J. Dart (eds.). "Biological Nitrogen Fixation in
Farming Systems of the Tropics." Chichester, John Wiley and Sons. pp.
45-60.
RAO, R.V. and D.L.
KEISTER (1978). Infection threads in
root hairs of soybean (Glycine max) plants inoculated with Rhizobium
japonicum. Protoplasma 97: 311-316.
REYES, V.G. and SCHMIDT,
E.L. (1981). Populations of Rhizobium japonicum associated with the
surfaces of soil-grown roots. Plant
Soil 61: 71-80.
REYES, V.G. and SCHMIDT,
E.L. (1979). Population densities of Rhizobium japonicum strain
123 estimated directly in soil and rhizospheres. Applied and Environ. Microbiol. 37: 854-858.
ROBERT, F.M. and SCHMIDT,
E.L. (1983). Population changes and persistence of Rhizobium phaseoli in soil
and rhizospheres. Applied and Environ. Microbiol. 45: 550-556.
ROBINSON, A.C. (1967).
The influence of host on soil and rhizosphere populations of clover and
lucerne root nodule bacteria in field. J. Aust. Inst. Agric. Sci. 33: 207-209.
ROBSON, A.D. (1978).
Mineral nutrients limiting Nitrogen Fixation in legumes. In: C.S. Andrew
and E.J. Kamprath (eds.). Mineral nutrition of legumes in tropical soils. CSIRO
Press, Melbourne. pp. 277-294.
ROSENDAHL, L. (1984).
Rhizobium strain effects on yield and bleeding sap amino
compounds in Pisum sativum.
Physiologia Plantarum 60: 215-220.
ROVIRA, A.D. (1961).
Rhizobium number in the rhizosphere of red clover and paspalum in
relation to soil treatment and the numbers of bacteria and fungi. Austr. J. Agric. Research 12: 77-83.
RUSSEK, E. and CALDWELL,
R.R. (1983). Computation of most
probable numbers. Applied and Environ.
microbiol. 5(5): 1646-1950.
SANCHEZ, P.A. (1976).
"Properties and Management. of Soil in the Soil in the
Tropics." Pbl. John Wiley and
Sons, N.Y.
SAYED, K.M. (1979).
"Effect of Soil Type, Levels of Salinity and Application of
Ammonium Sulphate on Symbiotic Relationship between Root Nodule Bacteria and
Soybean Plants." M.Sc. Thesis, Univ. of Tanta, Kafr. El-Sheikh, Egypt.
SEARLE, P.G.E., COMUNDAN,
Y., SHEDDEN, D.C. and NAUCE, R.A.
(1981). Field Crop. Res. 1:
133-145.
SEN, A.N. (1958).
Nitrogen economy of soil under rahar (Cajanus cajan). J. Indian. Soc. Soil Sci. 6: 171-176.
SINGH, S.R. (1981).
Evaluation of electrodyn Sprayer.
International Institute of Tropical Agriculture (IITA), Ibadan, Nigeria,
1981 Annual Report. p. 137.
SINGLETON, P.W. and
STOCKINGER, K.R. (1983). Compensation against ineffective nodulation
in soybean. Crop Science 23: 69-72.
SOMASEGARAN, P. and
HOBEN, J.H. (1985). "Methods in Legume-Rhizobium Technology. NIFTAL Project and MIRCEN, Paia Hawaii. p.
367.
STEEL, R.G.D., and J.H.
TORRIE (1960). "Principles and
Procedures of Statistics with Special Reference to the Biological
Sciences." McGraw-Hill Book Co., New York.
SUNDARA RAO, W.V.B. (]971).
Field experiments on nitrogen fixation by nodulated legumes. In. T.A.
Lie and E.G. Mulder (eds.). "Biological Nitrogen Fixation in Natural and
Agricultural Habitants." Plant and Soil, Spec. Vol. pp. 287-291.
SUTTON, W.D. (1983).
Nodule development and Senescence. In: Broughton W.J. (ed.).
"Nitrogen Fixation of 3 Legumes." Clarendon Press, Oxford, U.K. PP.
144-212.
TIMM, J.A. (1944). "General Chemistry" (1st Edition.
4th Impression). McGraw Hill Book Comp. Inc. New York and London. p. 403.
TRINICK, M.J. (1982).
Host-Rhizobium Associations. In: "Nitrogen Fixation in
Legumes." J.M. Vicent (ed.). United Kingdom Edition by Acad. Press, Inc.
(London) Ltd. 24/28 Oral Road Lond. NWI 7DX. Pp. 111-122.
TUZIMURA, K. and
WATANABE, I. (1961). Estimation of number and root- nodule
bacteria by nodulation-dilution frequency method. Soil Sci. Plant Nutrition, 7. 61-65.
TUZIMURA, K. and
WATANABE, I. (1962). The effects of Rhizosphere of various plants
on the growth of Rhizobium.
Ecological studies of Root-nodule bacteria. Part 3. Soil Sci. Plant
Nutrition (Tokyo) 8: 153-157.
VANDECAVEYE, S.C. and
MOODIE, C.D. (1943). Effects of Rhizobium meliloti
bacteriophage on alfalfa. Proc. Soil
Sci. Soc. Am. 8: 241-247.
VANDECAVEYE, S.C.,
FULLER, W.H. and KATZNELSON, H. (1940).
Bacteriophage of rhizobia in relation to symbiotic nitrogen fixation by
alfalfa. Soil Sci. 50: 15-17.
VINCENT, J.M. (1977). Rhizobium: general microbiology. In:
R.W.F. Hardy and Silver "A Treatise on Dinitrogen Fixation". Vol. III, Biology. Wiley,
New York pp. 277-354.
VINCENT, J.M. (1974).
Root nodule symbiosis with Rhizobium. In: "The Biology of
Nitrogen Fixation." A, Quispel
(ed.). North-Holland Research diomographs frontiers of Biology. Pp. 265-341.
VINCENT, J.M. (1982).
The legume Rhizobium symbiosis. In: "Nitrogen Fixation in
Legumes." J.J. Vicent (ed.).
Academic Press, Inc. (London) Ltd. 24/28. Oral Road Lond. NWI 7DX. pp.
1-4.
VINCENT, J.M.
(1965). Environmental factors in the
fixation of Nitrogen by the legume. In: "Soil Nitrogen" W.N.
Bathlomew and F.E. Clark (eds.). Monograph 10, Amer. Soc. of Agronomy, Madison,
Wisconsin. pp. 384-435.
VINCENT, J.M. (1970).
"A manual for the practical study of root-nodule bacteria."
IB. P Handbook No.15.
VIRTANEN, A.I., JORMA,
J., LINKOLA, H. and LINNASALMI, A.
(1947). The relation between Nitrogen fixation and leghaemoglobin
content of leguminous root-nodules. Acta. Chem. Scan. 1: 90-111.
WALKLEY, A. (1947).
A critical examination of a rapid method for determining
carbon in soils. Effect of variation in
digestion conditions and of inorganic soil constituents. Soil Sci. 63: 251-264.
WEAVER, R.W. and
FREDRICK, L.R. (1982). Rhizobium. In: Black, C.A. and Evans, E.E.
(eds.) "Methods of soil analysis" Part 2. Chemical and
microbiological properties. Agron. Mono. 9: 1043-1070.
WEAVER, R.W., FREDRICK,
L.K. and DUMENIL, L.C. (1972). Effect of soybean cropping and soil
properties on numbers of Rhizobium japonicum in Iowa Soils. Soil Sci. 114: 137-141.
WEST, P. (1939).
Excretion of thiamin and biotin
by the roots of higher plants. Nature, London. 144: 1050-1051.
WETSELAAR, R., JAKOBSEN,
J. and CHAPLIN, G.R. (1973). Nitrogen
balance in crop systems in tropical Australia. Soil Biol. Biochem. 5: 35-40.
WILCOX, J.R. (1987).
Soyabeans: Improvement, production and uses. Agron. J. 16: Second
Edition.
WILSON, J.R. (1970).
Response to salinity in Glycine. VI. Some effects of a range of
short-term salt. stresses on the growth, nodulation and Nitrogen fixation of Glycine
wightii (formerly javanica).
Aust. J. Agric. Res. 21: 571-582.