Back to Dr. N. V. Hue
Land Application of Biosolids
N. V. Hue
Professor of Environmental Soil Chemistry
University of Hawaii
Abstract
Production, treatment, and properties of biosolids are discussed, with attention
given to Hawaii's products from waster-water treatment plants at Sand Island, Honouliuli,
and Kaneohe (Mokapu). Implications of the EPA's 503 rule and impacts of biosolids
application to land are examined in terms of soil fertility, crop uptake, water quality, and
human health.
Production of Biosolids
Let me begin by asking you a simple question: how much water, on
average, do you think you use in a day? 50 gallons, one hundred gallons, or 200
gallons? How many times a day do you flush the toilet? take a shower and
clean dishes? The correct answer is about 100 gallons/day. In fact, recent
statistics show that Oahu with about 0.8 million people currently generates 133
million gallons of waste water a day, 60% of that or 80 mill. gal. come from
residential use.
And as you all know, the Clean Water Act requires that any waste water
must be treated to protect human health and the environment before being
discharged or reused. As a result, the quantity of waste water residues, now
called biosolids but commonly known as sewage sludge, has increased many
fold. For example, in 1983 the U.S. generated 6.2 million dry metric tons of
biosolids, corresponding roughly to 26 kg per person per year. Biosolids
production increased to 9 million ton/year in 1995 and is expected to reach at
least 11 million ton/year by the year 2000 as the population grows and more
effective wastewater treatment processes are implemented.
In Hawaii, we produce approximately 40 thousand dry tons of biosolids a
year. What can we do with them?
Well before we address that question, let's take a quick look at how
biosolids are treated and handled, and how safe these materials are.
Treatment of Biosolids
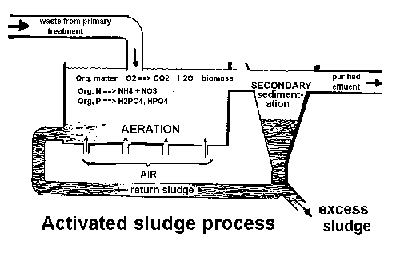 Once pumped into a treatment plant, waste water is first held in a large
clarifying tank to remove readily settleable solids. This treatment process
produces primary biosolids which contain 3 to 7% solids and can be easily
thickened and de-watered. Each thousand gallons of wastewater produce about
3 gallons of primary biosolids. The clarified wastewater may further undergo
secondary treatment, which often involves biological processes such as seeding
or re-circling biosolids into the wastewater stream as shown here.
Once pumped into a treatment plant, waste water is first held in a large
clarifying tank to remove readily settleable solids. This treatment process
produces primary biosolids which contain 3 to 7% solids and can be easily
thickened and de-watered. Each thousand gallons of wastewater produce about
3 gallons of primary biosolids. The clarified wastewater may further undergo
secondary treatment, which often involves biological processes such as seeding
or re-circling biosolids into the wastewater stream as shown here.
In the holding tank,
{CH20} + 02 ==> CO2 (gas) + H2O + microbial biomass
organic N ==> NH4 + NO3-
organic P ==> H2PO4- + HPO42-
This secondary treatment produces secondary or activated biosolids
containing 0.5 - 2.0% solids, which are harder to thicken and de-water than the
primary biosolids.
Both primary and secondary biosolids are odorous and pathogen rich.
They must be stabilized to reduce odor potential and pathogen levels.
Major stabilization processes include anaerobic digestion, aerobic
digestion, lime addition and composting. (1) Anaerobic digestion is the most
common process, by which biosolids are retained in the absence of air for at
least 60 days at 20 C or 15 days at 35 - 55C. This process can bio-degrade 40
- 50% of the volatile solids in the sludge by converting org. carbon to CH4 and
org. N to aqueous NH3. (2) Aerobic digestion is conducted by agitating biosolids
with air or oxygen to maintain aerobic conditions for 60 days at 15C, 40 days at
20C or 10 days at 55 - 60C. Volatile solids are also reduced significantly by
this process, but not as much as in the anaerobic digestion. (3) Lime
stabilization involves the addition of sufficient CaO or Ca(OH)2 to biosolids such
that pH 12 must be reached after 2 hours of contact. This process would
precipitate most heavy metals as well as Ca and phosphate. The high pH also
kills most pathogens. (4) Biosolids composting is a process in which biosolids
are mixed with a bulking agent, such as wood chips, saw dust or yard waste and
allowed to decompose aerobically for several weeks. If operated properly, the
temperature in the composting mixture can rise to 55 - 60c and remains hot for
several days, effectively killing most pathogens, weed seeds, and parasites.
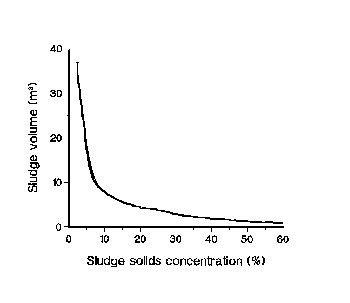
After stabilization, biosolids are usually dewatered to
save transportation and storage costs. Sludge volume can
be reduced substantially by partially eliminating its water. For
example, raising the solids content from 5 to 10% cuts the
sludge volume by half. On the other hand, weight reduction
is the primary interest when the solids content exceeds 30%.
Dewatering can be accomplished through centrifugation, vacuum filter,
filter press, sand bed drying, or heat drying. Dewatering produces a cake with 15
to 25% solids by centrifugation or vacuum filter, 35 to 50% solids by filter press,
50 to 80% solids by air drying, and 90 to 99% solids by heat drying.
In Hawaii, the two largest wastewater treatment plants at Sand Island and
Honouliuli produce primary biosolids with about 25% solids after centrifugation,
the Kaneohe (Mokapu) plant produces anaerobically digested biosolids with
about 20% solids. Two smaller plants in Waimanalo and Waianae produce
secondary digested biosolids with 60 to 80% solids after sand bed drying (Table 1).
Table 1. Waste-water and sludge treatment on Oahu, Hawaii.
|
Plant |
Digestion |
Dewatering |
Solids, % |
Dry ton/year |
|
Sand Island |
Undigested |
centrifuge |
25 - 30 |
25,000 |
|
Honouliuli |
Undigested |
centrifuge |
25 - 30 |
6,500 |
|
Kaneohe |
anearobic |
centrifuge |
17-22 |
3,500 |
|
Waimanalo |
aerobic |
dry beds |
60 - 80 |
70 |
|
Waianae |
anaerobic |
dry beds |
60 - 80 |
60 |
Properties of Biosolids.
The next question would come to our mind is that what kind of biosolids do
we get? Are they safe to use or even to dispose of? To partially answer that
question, let's look at the composition of some biosolids. Pathogens, and to a
lesser extent toxic organic chemicals, are important factors in dealing with
biosolids. Unfortunately, I have no expertise in those areas. So I will limit the
rest of my talk to plant nutrients and heavy metals in biosolids.
Plant Nutrients
As Table 2 shows, plant nutrients such as N, P, K, Ca, Mg in
biosolids vary widely. My analysis of Oahu's biosolids in 1991-1992 shows that
total N ranges from 1.3 to 6.3%, P from 0.3 to 0.9%, K from 0.01 to 0.1% and so
on.
Table 2. Nutrient contents in biosolids
|
Variable |
|
Total nutrients, |
% dry weight |
|
|
|
|
N |
P |
K |
Ca |
Mg |
|
|
Hawaii survey, |
1991 - 1992 |
(22 samples) |
|
|
|
Range |
1.32 - 6.25 |
0.26 - 0.85 |
0.01 - 0.13 |
0.50 - 6.81 |
0.08 - 0.80 |
|
Mean |
3.8 |
0.6 |
0.06 |
1.78 |
0.29 |
|
|
New York survey, |
1985 |
(15 samples) |
|
|
|
Range |
1.19 - 4.93 |
0.22 - 3.13 |
0.03 - 0.46 |
0.32 - 15.9 |
0.04 - 0.81 |
|
Mean |
2.9 |
1.2 |
0.19 |
3.92 |
0.35 |
|
|
Michigan survey, |
1980 |
(> 200 samples) |
|
|
|
Range |
0.2 - 21.0 |
0.1 - 15.0 |
0.1 - 6.5 |
0.5 - 17.0 |
0.1 - 2.5 |
|
Mean |
3.5 |
2.2 |
0.5 |
4 |
0.7 |
|
"Typical" sludge |
3.2 |
1.4 |
0.23 |
2.7 |
0.4 |
Generally, digested or secondary biosolids contain higher nutrient content
than raw or primary biosolids because much of the volatile organic matter has
been given off as CH4 or CO2 during the digestion process. For example, our
Hawaii work shows that the Sand Island and Honouliuli biosolids, which are
primary, contain only 1.60% and 2.0 %N whereas the Kaneohe, Waimanalo, and
Waianae biosolids, which are anaerobically digested, contain 5.24%N (Table 3).
Table 3. Total nutrient contents in some Hawaii's biosolids
|
Biosolids (digest. Proc.) |
|
Nutrient conc., |
% dry weight |
|
|
|
(digest. Proc.) |
N |
P |
K |
Ca |
Mg |
|
Sand island (primary) |
1.6 |
0.36 |
0.36 |
0.7 |
0.13 |
|
Honouliuli (primary) |
2.02 |
0.57 |
0.014 |
0.9 |
0.1 |
|
Kaneohe (anaerobic) |
5.24 |
0.59 |
0.063 |
1.53 |
0.32 |
|
Waimanalo (aerobic) |
6.1 |
0.82 |
0.04 |
2.64 |
0.28 |
|
Waianae (anaerobic) |
3.54 |
0.47 |
0.13 |
1.42 |
0.34 |
By averaging all available data together, a so called "typical" biosolids
would have 3.2% N, 1.4% P, or 3.1 % P2O5, 0.23% K, 2.7% Ca and 0.4% Mg.
This information presents several interesting points. (i) First, with respect to N
content, biosolids are comparable to chicken manure and could be used as a low
grade, slow release N fertilizer, (ii) second, proportion of P to N in biosolids is
about one half to one fourth, which is much higher than the P/N ratio in plants. In
plants, the P to N ratio is about one fifth to one tenth. Thus, if we use biosolids
to meet plant's N requirement, often time we would exceed plant's P needs.
Over time, P may build up to a point that P pollution is a strong possibility. (iii)
Finally, potassium content of biosolids is inherently low because most K
compounds are water soluble and remain in the sewage effluent or the aqueous
fraction during biosolids dewatering. Thus, supplemental K fertilizers are likely
needed for K poor soils, if the biosolids are to be used as an organic fertilizer.
Heavy Metals.
In my opinion, heavy metal content is the most important factor
determining the use of biosolids. And I think the EPA would agree with that
view, as the 503 regulation shows. In this rule, 10 metals are
regulated. They are arsenic (As), cadmium (Cd), chromium (Cr), Copper (Cu), lead (Pb), mercury (Hg), Molybdenum (Mo), nickel (Ni), selenium (Se), and zinc (Zn).
Table 4. EPA's pollutant limits for land applied biosolids
|
Pollutants |
Ceiling Conc. |
Conc. Limits for "clean" sludges |
|
|
mg/kg |
mg/kg |
|
As |
75 |
41 |
|
Cd |
85 |
39 |
|
Cr |
3000 |
1200 |
|
Cu |
4300 |
1500 |
|
Pb |
840 |
300 |
|
Hg |
57 |
17 |
|
Mo |
75 |
18 (?) |
|
Ni |
420 |
420 |
|
Se |
100 |
36 |
|
Zn |
7500 |
2800 |
The middle column lists the maximum concentrations of the ten metals that
biosolids must not exceed if land application is the intended use. Biosolids
whose metal concentrations fall in between these 2 limits can be land-applied
but with some restrictions.
In contrast, biosolids whose metal concentrations are below those listed in the
right most column can be land-applied as a soil amendment or fertilizer with
minimal restrictions; and these restrictions concern only with pathogens. Land
applied biosolids must meet either class A or class B pathogen requirements.
Class A biosolids have nearly undetectable bacteria counts, meaning that the
density of fecal coliform must be less than 1000 most probable numbers (MPN)
per gram of dry biosolids or that the density of salmonella sp. must be less than
3 in 4 grams of dry biosolids. For class B biosolids, the density of fecal coliform
must be less than 2 x 106 MPN/gram biosolids. To protect public health, the
EPA has put several site restrictions on class B biosolids, when they are applied
to land. For example, animals must be kept from grazing for at least 30 days,
and food crops must not be harvested for at least 14 months (and in some cases
as long as 38 months) after biosolids application.
How does the EPA's 503 rule affect Hawaii's biosolids? Frankly not much,
at least in the short term. For one thing, our state has not seriously considered
using biosolids on land, perhaps because of negative public perception about bio
solids. For another, Hawaii's biosolids are fairly low in heavy metals, they would
likely meet the exceptional quality (EQ) or clean biosolids category set by the
EPA.
Table 5. Total heavy metals in some Hawaii biosolids
|
Sludge |
Cd |
Cu |
Mn |
Ni |
Zn |
|
|
mg/kg |
mg/kg |
mg/kg |
mg/kg |
mg/kg |
|
Sand island |
3.15 |
220 |
372 |
38.5 |
421 |
|
Honouliuli |
4.79 |
317 |
44 |
24.6 |
494 |
|
Kaneohe |
5.21 |
399 |
53 |
29.5 |
869 |
|
Waimanalo |
10.5 |
426 |
109 |
42.3 |
1038 |
|
Waianae |
3.13 |
462 |
609 |
41.3 |
520 |
|
EPA's clean sludge |
39 |
1500 |
|
420 |
2800 |
|
"Typical" US sludge |
8 |
410 |
260 |
45 |
678 |
As shown in Table 5, our biosolids contain about one half to one tenth
the pollutant limits for the clean biosolids category. However, don't forget that
most US biosolids are similarly clean. The median concentrations of heavy
metals in U.S. biosolids are 8 ppm for Cd, 410 ppm for Cu, 260 ppm for Mn, 45
ppm for Ni and 700 ppm for Zn.
Impacts of Biosolids application to land on
soil fertility, crops uptake, water quality, and human health
Except in Hawaii, about 50% of US biosolids are land applied, according to
a recent EPA report. In some states, such as Colorado, Florida, Oregon, and
Washington, this percentage has passed 70%. Nitrogen is often assumed to be
the main benefit from biosolids. Thus, the application rate is usually calculated
based on the N need of a crop. You then would ask: How much would be a
reasonable or agronomic rate? Well, it varies with the crop that you're going to
grow and the quality of your biosolids. I can give you an example. Let's use corn
as your favorite crop. A corn crop needs 200 kg N/ha for good growth. Now we
want to supply that N from an anaerobically digested biosolid containing 3% total
N of which 0.5% is inorganic (NH4 + NO3)-N, the remaining is organic N. A few
facts and assumptions are needed for the calculation.
(1) All inorganic N is readily available for crop uptake.
(2) Organic N must be transformed into inorganic N before plants can take up
that N. The rate of this transformation which we call N mineralization
depends on many factors: sludge quality, soil properties, temperature, and
moisture. In warm and humid regions like Hawaii, it is reasonable to
assume that:
For primary and aerobically digested biosolids: 40% of organic N
would become plant available during the first year after application.
anaerobically digested or chemically stabilized biosolids: 20%
composted biosolids: 10%
biosolid: 3.0% total N & 0.5% (NH4 + NO3) -N Target: 200 Kg
N/ha
(anaerobic) 2.5% organic N
200/(0.005 + 0.2 x 0.025) = 20,000 Kg /ha
20 ton/ha
So our answer would be about 20 ton/ha. Generally, annual application
rates of biosolids to agricultural land range from 5 to 70 ton/ha, with 15 ton/ha
being typical.

If your soil is poor in plant nutrients as those soils in central Oahu, then an
application of biosolids would make plant grow much better as shown here for a
red soil in Wahiawa. Choi Sum grew much better in the plot receiving 45 ton/ha
biosolids than the control (Slide). In fact, the beneficial effect is still noticeable
more than 10 years after we applied the biosolids. Indeed, we have identified
that interactions between organic matter in the biosolids and soil minerals are
the cause of these benefits. For example, plants can use P fertilizers recently
applied to biosolids-amended plots more effectively than those applied to the
control.
However, you need to know a bit about your soil, biosolids, as well as the
crop you're going to grow. If you use a biosolid that's low in N and high in C/N
ratio such as the Sand Island biosolid and the crop is a fast growing one like
tomato, then the presence of biosolids may even hurt your crop in the short term
because of N immobilization as shown here.
 The left pot is the control with no fertilizer added; the center received 2.5% Sand Island biosolid by weight,
which is equivalent to about 45 ton/ha, and the right pot received 2.5%
Kaneohe biosolid. You can see the difference in growth.
The left pot is the control with no fertilizer added; the center received 2.5% Sand Island biosolid by weight,
which is equivalent to about 45 ton/ha, and the right pot received 2.5%
Kaneohe biosolid. You can see the difference in growth.
On the other extreme, you may ask what would
happen if so much biosolids are applied that more N is
released than crops can use? Especially with repeated
biosolid applications year after year. Of course, through
biological transformation, most of organic N in the
biosolids would end up as NO3-.
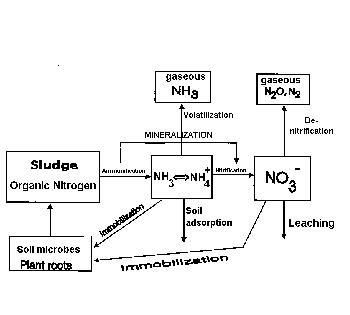 NO3 leaching and
ground water pollution is a justifiable concern. Most of
our soils carry negative charge, the same as NO3-. The
two tend to repel each other, meaning that NO3
movement in soil is fairly fast. Hawaii soils with high
clay content and many micropores, retart NO3
movement somewhat. Nevertheless, NO3- does move downward.
NO3 leaching and
ground water pollution is a justifiable concern. Most of
our soils carry negative charge, the same as NO3-. The
two tend to repel each other, meaning that NO3
movement in soil is fairly fast. Hawaii soils with high
clay content and many micropores, retart NO3
movement somewhat. Nevertheless, NO3- does move downward.
Why are we so concerned about nitrate pollution of ground water? In my
opinion, that concern comes partially from the fear that if nitrate can move to
ground water then something else like pesticides can too. In reality, nitrate itself
is not toxic to living organisms but nitrite (NO2-) is. In babies of 6 months old or
younger, their stomach has certain bacteria that can convert NO3- to NO2- .
Nitrite can then oxidize Fe2+ in hemoglobin to Fe3+ , converting hemoglobin to
methemoglobin which is a poor O2 carrier. As the O2 carried by the blood
decreases, the body is suffocated and turns blue. Thus, the name "blue-baby"
syndrome came about. While most infants can tolerate NO3- in water at levels
much higher than 10 mg N/L, which is the EPA's standard for drinking water,
some infants begin to exhibit methemoglobin symptoms at levels only slightly
higher than 10 mg N/L after a few days of consumption. Adults and older
children are much less susceptible to MeHb problem because stomach HCl
levels increase with age, and HCl kills most of the bacteria that can reduce NO3-
to NO2-. For adults, 10 mg NO3-N/L is a lifetime health advisory level. The
rationale for this advice is that, although NO3- itself is not carcinogenic, a small
fraction of it could be reduced to NO2- and react with secondary and tertiary
amines in the human body to form nitrosamines. Nitrosamines have been
identified as a potent carcinogen in animals and may be an important carcinogen
in humans. However, there is no conclusive evidence linking any type of cancer
to NO3- in drinking water.
Heavy Metals
What about heavy metals? Unlike nitrogen and P, which is an asset,
heavy metals are a liability of biosolids. It is interesting to note that before 1993
when the EPA's 503 rule came into effect, only Cd was regulated in biosolid use.
Now 10 metals in biosolids are regulated. Because of time constraints, we're
going to focus only on Cd and Mo, in this discussion.
Two important factors that we should be aware of when working with heavy
metals in biosolids and in soils.
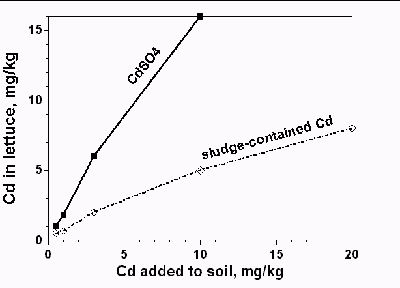 1. Pound for pound, the bio-availability of metals in
biosolids is much lower than that of a pure metal
salt, say sludge-contained Cd vs CdCl2 or
CdSO4. (Slide). The reason is that most metals
in biosolids are in solid forms with very low
solubility. They are either precipitated by
carbonate and/or sulfide, complexed by organic
matter, or sorbed by Fe, and Mn oxides. The
ceiling concentration limits for the 10 heavy
metals in the 503 rule, that we discussed earlier, were set as such
partially because the EPA worried that when heavy metals in
biosolids are high enough, they may act like a metal salt, thus cause
severe toxicity to plants and animals.
1. Pound for pound, the bio-availability of metals in
biosolids is much lower than that of a pure metal
salt, say sludge-contained Cd vs CdCl2 or
CdSO4. (Slide). The reason is that most metals
in biosolids are in solid forms with very low
solubility. They are either precipitated by
carbonate and/or sulfide, complexed by organic
matter, or sorbed by Fe, and Mn oxides. The
ceiling concentration limits for the 10 heavy
metals in the 503 rule, that we discussed earlier, were set as such
partially because the EPA worried that when heavy metals in
biosolids are high enough, they may act like a metal salt, thus cause
severe toxicity to plants and animals.
 2. Except for Mo, most other heavy metals are
more water soluble and thus more bio-available when soil pH decreases (that is
when the soil becomes more acidic). As
shown here for Zn (slide), at pH 7, the limit of
1 mg Zn/L in soil solution, a level that may
cause slight reduction in growth of some
vegetables, would be attained at about 1200
ppm Zn in soil. At pH 6, that soluble Zn level
would be reached with 100 ppm Zn in soil.
And at pH 5, it takes only 40 ppm Zn in soil to maintain 1 ppm Zn in
solution. That's why it is recommended that soil pH be adjusted to
between 6.5 and 7.0 when biosolids are applied and be maintained at
6.0 or above as long as the land is used for production of crops for
human or animal consumption.
2. Except for Mo, most other heavy metals are
more water soluble and thus more bio-available when soil pH decreases (that is
when the soil becomes more acidic). As
shown here for Zn (slide), at pH 7, the limit of
1 mg Zn/L in soil solution, a level that may
cause slight reduction in growth of some
vegetables, would be attained at about 1200
ppm Zn in soil. At pH 6, that soluble Zn level
would be reached with 100 ppm Zn in soil.
And at pH 5, it takes only 40 ppm Zn in soil to maintain 1 ppm Zn in
solution. That's why it is recommended that soil pH be adjusted to
between 6.5 and 7.0 when biosolids are applied and be maintained at
6.0 or above as long as the land is used for production of crops for
human or animal consumption.
We're concerned about heavy metals because at elevated levels they are harmful
to humans and animals. Let's use Cd as an example since this metal has been
studied most. The potential risk of high Cd in food to human health was first
recognized in the early 70's when Cd-related "itai-itai" disease was reported. In
the 60's a large number of Japanese farmers suffered Cd health effects after
long-term consumption of Cd enriched rice grown in paddies irrigated with Cd
polluted water (from the Jinzu river). Among those farmers, there was a strong
correlation between kidney problems, such as high levels of proteins and sugars
in urine, and Cd concentration in rice they ate. By the way, the name "itai-itai",
meaning ouch-ouch in English, came from expressions of pain by women
suffering Cd-induced bone fractures.
Cd accumulates most in kidneys, and to a lesser extent in liver, with a
mean residence time of about 30 years. Thus, the early health effect of
excessive chronic Cd exposure is the renal dysfunction or kidney disease. It is
believed that much of the physiological effect of Cd arises from its chemical
similarity to Zn. Cd may replace Zn in some enzymes, thereby altering the
stereostructure of the enzyme and impairing its catalytic activity.
Molybdenum is another interesting story. At low concentrations (say, less
than 5 ppm in plant biomass), Mo is an essential nutrient for plants and animals.
Yet, at high concentrations in forage, Mo can poison grazing animals. Ingested
Mo is transformed in the rumen to tetrathiomolybdate, which binds Cu strongly,
preventing Cu absorption by the gut and reducing Cu bio-availability. If forages
contain 5 to 10 ppm Mo such that Cu/Mo ratio is less than 2, induced Cu
deficiency may occur. Generally, the availability of Mo for plant uptake increases
and that of Cu decreases as soil pH rises. Thus, Mo toxicity to animals is most
frequently associated with forage grown on alkaline soils.
Concluding Remarks
We have discussed biosolids treatment and properties as well as their
impacts -- both good and bad -- on man and the environment. In conclusion,
the following points should be emphasized.
On the positive side: (1) biosolids N, which is mostly organic, is less likely
to cause ground water pollution than chemical N fertilizers. (2) Other nutrients,
particularly P, are also valuable. In some soils, such as Fe-deficient calcareous
soils, biosolids can provide many essential micro nutrients (e.g. Fe, Zn, Cu) even
more effectively than commercial fertilizers. (3) The organic matter in biosolids
can adsorb and deactivate heavy metals to such an extent that an exceptional
quality class of biosolids has been legally established.
On the negative side: biosolids contain a "soup" of metals, organic
chemicals and pathogens. Most of these pollutants have no known adverse
effects, but some show inconclusive, thus worrisome effects, while a few have
been proven hazardous to human health or the environment. Also, economically
the build-up of heavy metals from land-applied biosolids may devaluate the land
in the future. Obviously, risks are involved in using biosolids on land. A delicate
balance must be sought between the needs for re-use or disposal of biosolids
and the responsibility to protect public health and the environment.
But, if other states can do it, so can Hawaii. Right?
Back to Dr. N. V. Hue
 Once pumped into a treatment plant, waste water is first held in a large
clarifying tank to remove readily settleable solids. This treatment process
produces primary biosolids which contain 3 to 7% solids and can be easily
thickened and de-watered. Each thousand gallons of wastewater produce about
3 gallons of primary biosolids. The clarified wastewater may further undergo
secondary treatment, which often involves biological processes such as seeding
or re-circling biosolids into the wastewater stream as shown here.
Once pumped into a treatment plant, waste water is first held in a large
clarifying tank to remove readily settleable solids. This treatment process
produces primary biosolids which contain 3 to 7% solids and can be easily
thickened and de-watered. Each thousand gallons of wastewater produce about
3 gallons of primary biosolids. The clarified wastewater may further undergo
secondary treatment, which often involves biological processes such as seeding
or re-circling biosolids into the wastewater stream as shown here.


 The left pot is the control with no fertilizer added; the center received 2.5% Sand Island biosolid by weight,
which is equivalent to about 45 ton/ha, and the right pot received 2.5%
Kaneohe biosolid. You can see the difference in growth.
The left pot is the control with no fertilizer added; the center received 2.5% Sand Island biosolid by weight,
which is equivalent to about 45 ton/ha, and the right pot received 2.5%
Kaneohe biosolid. You can see the difference in growth.  NO3 leaching and
ground water pollution is a justifiable concern. Most of
our soils carry negative charge, the same as NO3-. The
two tend to repel each other, meaning that NO3
movement in soil is fairly fast. Hawaii soils with high
clay content and many micropores, retart NO3
movement somewhat. Nevertheless, NO3- does move downward.
NO3 leaching and
ground water pollution is a justifiable concern. Most of
our soils carry negative charge, the same as NO3-. The
two tend to repel each other, meaning that NO3
movement in soil is fairly fast. Hawaii soils with high
clay content and many micropores, retart NO3
movement somewhat. Nevertheless, NO3- does move downward.
 1. Pound for pound, the bio-availability of metals in
biosolids is much lower than that of a pure metal
salt, say sludge-contained Cd vs CdCl2 or
CdSO4. (Slide). The reason is that most metals
in biosolids are in solid forms with very low
solubility. They are either precipitated by
carbonate and/or sulfide, complexed by organic
matter, or sorbed by Fe, and Mn oxides. The
ceiling concentration limits for the 10 heavy
metals in the 503 rule, that we discussed earlier, were set as such
partially because the EPA worried that when heavy metals in
biosolids are high enough, they may act like a metal salt, thus cause
severe toxicity to plants and animals.
1. Pound for pound, the bio-availability of metals in
biosolids is much lower than that of a pure metal
salt, say sludge-contained Cd vs CdCl2 or
CdSO4. (Slide). The reason is that most metals
in biosolids are in solid forms with very low
solubility. They are either precipitated by
carbonate and/or sulfide, complexed by organic
matter, or sorbed by Fe, and Mn oxides. The
ceiling concentration limits for the 10 heavy
metals in the 503 rule, that we discussed earlier, were set as such
partially because the EPA worried that when heavy metals in
biosolids are high enough, they may act like a metal salt, thus cause
severe toxicity to plants and animals.
 2. Except for Mo, most other heavy metals are
more water soluble and thus more bio-available when soil pH decreases (that is
when the soil becomes more acidic). As
shown here for Zn (slide), at pH 7, the limit of
1 mg Zn/L in soil solution, a level that may
cause slight reduction in growth of some
vegetables, would be attained at about 1200
ppm Zn in soil. At pH 6, that soluble Zn level
would be reached with 100 ppm Zn in soil.
And at pH 5, it takes only 40 ppm Zn in soil to maintain 1 ppm Zn in
solution. That's why it is recommended that soil pH be adjusted to
between 6.5 and 7.0 when biosolids are applied and be maintained at
6.0 or above as long as the land is used for production of crops for
human or animal consumption.
2. Except for Mo, most other heavy metals are
more water soluble and thus more bio-available when soil pH decreases (that is
when the soil becomes more acidic). As
shown here for Zn (slide), at pH 7, the limit of
1 mg Zn/L in soil solution, a level that may
cause slight reduction in growth of some
vegetables, would be attained at about 1200
ppm Zn in soil. At pH 6, that soluble Zn level
would be reached with 100 ppm Zn in soil.
And at pH 5, it takes only 40 ppm Zn in soil to maintain 1 ppm Zn in
solution. That's why it is recommended that soil pH be adjusted to
between 6.5 and 7.0 when biosolids are applied and be maintained at
6.0 or above as long as the land is used for production of crops for
human or animal consumption.