|
|||||
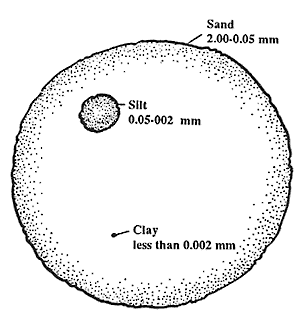
Soil MineralsSoil minerals play a vital role in soil fertility since mineral surfaces serve as potential sites for nutrient storage. However, different types of soil minerals hold and retain differing amounts of nutrients. Therefore, it is helpful to know the types of minerals that make up your soil so that you can predict the degree to which the soil can retain and supply nutrients to plants. There are numerous types of minerals found in the soil. These minerals vary greatly in size and chemical composition. Soil Mineral Particle SizeParticle size is an important property that allows us to make distinctions among the different soil minerals. Soils contain particles that range from very large boulders to minute particles which are invisible to the naked eye. To further distinguish particles based upon size, particles are separated into the two categories: the coarse fraction and the fine earth fraction. Fine Earth FractionWhen we refer to most soils of Maui, we are generally referring to the second category of particle size: the fine earth fraction. This is because the soils of Maui are almost exclusively finely textured. The fine earth fraction includes any particle less than 2.0 mm (.078 inches) and is divided into three classes of size: sand, silt, or clay. To put this into perspective, the width of the lead in a No. 2 pencil is approximately 2.0 mm. Table 1 provides descriptions of each class in the fine earth fraction. Table 1. Description of sand, silt, and clay classes.
Coarse FractionThe coarse fraction of soil includes any soil particles greater than 2mm. The coarse fraction includes boulders, stones, gravels, and coarse sands. These are rocky fragments and are generally a combination of more than one type of mineral. We are not very concerned with the coarse fraction in soil since the soils of Maui County primarily fall into the fine earth fraction. Weathering of Soil Minerals and Change in Mineral CompositionWeathering is the principal process that acts upon the earth’s primary minerals to form the smaller and finer particles that we call “soil.” Maui County is an excellent place to observe the effects of weathering since it contains both slightly weathered and highly weathered soils. In terms of nutrient management, the process of weathering greatly influences the availability of plant nutrients. Initially, as soil particles begin to weather, primary minerals release nutrients into the soil. As these particles decrease in size, the soil is also able to retain greater amounts of nutrients. Ultimately, however, the capacity to hold and retain nutrients is greatly reduced in highly weathered soils, since most nutrients have been lost due to leaching. There are two types of weathering: physical weathering and chemical weathering. Differences in weathering patterns are the reason why there is a great range in soil particle size. Boulders are much less weathered than gravel. In return, gravel is much less weathered than clay particles. Clay particles may even weather into other materials, such as iron and aluminum oxides, which are generally resistant to further weathering. In the tropics, chemical weathering is very important. Since the climate is typically warm and moist year-round, it provides a suitable environment for continuous chemical weathering to occur. Over time, with sufficient amounts of rainfall and warm temperatures, mineral particles weather into smaller and smaller soil particles. As a result, tropical soils tend to be highly weathered soils. Table 2 provides a list of common primary, secondary minerals, aluminum and iron oxides, and amorphous materials in Hawaii. Physical WeatheringPhysical weathering is a process that breaks up and disintegrates parent rock, or primary minerals, within the earth. In the tropics, physical weathering is caused by the wetting and drying of rocks; erosion; actions of plants and animals; or the falling, smashing, or breaking of rock materials into smaller pieces. Chemical WeatheringChemical weathering is important in nutrient management since the resulting soil particles retain and supply nutrients. However, when highly weathered, the soil loses much of its nutrients due to excessive leaching. Thus, highly weathered soils tend to be infertile soils, while moderately weathered soils are generally more fertile. Once parent rock has broken down into smaller pieces, another process acts upon the rock. This process is chemical weathering. Chemical weathering involves the change, or transformation, of primary minerals into secondary minerals. Secondary minerals serve as the basic building blocks of the small particles with the soil. As a result, new materials may be synthesized, residual material may accumulate from materials (such as oxides) which cannot be furthered weathered, or materials can be lost as the result of leaching. Table 2. Important Primary Minerals and Weathered Materials on Maui
The following three links provide animated demonstrations of how the process of weathering transforms primary minerals into secondary minerals and other materials common to soils. These animations were created by North Carolina State University.
|
|||||||||||||||||||||||||||||||||||