|
|||||
PhosphorusIn the tropics, phosphorus is often the most limiting plant nutrient.
Phosphorus Forms and FunctionsForms of Phosphorus available for Plant Uptake
Functions of Phosphorus in PlantsPhosphorus is involved in many plant processes, including:
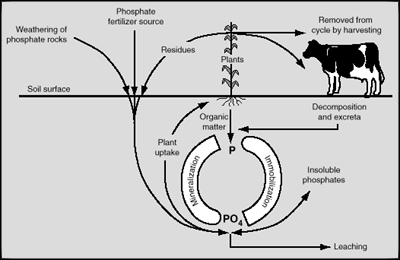
The Phosphorus CycleIn contrast to nitrogen, the atmosphere does not provide phosphorus. Instead, orthophosphates originate largely from primary and secondary minerals and/or from organic sources. However, the phosphorus cycle is by no means less complex than the nitrogen cycle, and there are many factors that affect the availability of phosphorus in the soil. The diagram below is an illustration of the phosphorus cycle.
Phosphorus Uptake by Plant RootsPlant roots absorb phosphorus from the soil solution. In comparison to other macronutrients, the phosphorus concentration in the soil solution is much lower and ranges from 0.001 mg/L to 1 mg/L (Brady and Weil, 2002). In general, roots absorb phosphorus in the form of orthophosphate, but can also absorb certain forms of organic phosphorus. Phosphorus moves to the root surface through diffusion. However, the presence of mycorrhizal fungi, which develop a symbiotic relationship with plant roots and extend threadlike hyphae into the soil, can enhance the uptake of phosphorus, as well especially in acidic soils that are low in phosphorus. For further information on mycorrhizal fungi and its use in Hawaii, click on the link below: Phosphorus Sorption and DesorptionP-sorption occurs when the orthophosphates, H2PO4- and HPO42-, bind tightly to soil particles. Since phosphate is an anion, particles that generate an anion exchange capacity will form strong bonds with phosphate. Particles with anion exchange capacity:
These particles are commonly found in many of the most highly weathered soils and high weathered volcanic soils of Hawaii. Since P-sorption results in a decrease of plant available phosphorus, P-sorption can become a major issue in many Hawaii soils. Additionally, in calcareous soils P-sorption may occur as phosphates sorb to impurities such as aluminum and iron hydroxides or displace carbonates in calcium carbonate minerals. Factors that affect P-sorption
Factors that decrease P-sorption:
Phosphate Precipitation and DissolutionPhosphate precipitation is a process in which phosphorus reacts with another substance to form a solid mineral. In contrast, dissolution of phosphate minerals occurs when the mineral dissolves and releases phosphorus. Precipitation and dissolution reactions greatly influence the availability of phosphate in the soil.
Solubility of Phosphate MineralsThe solubility of phosphate minerals is very dependent upon soil pH.
Mineralization and Immobilization of PhosphateIn an average soil, approximately 50% of total phosphorus is organic. Thus, soil organic phosphorus is a very important aspect of the P cycle. The various sources of organic phosphorus include
Like nitrogen, organic phosphorus is converted to inorganic phosphate through the process of mineralization. The immobilization of inorganic phosphate, in contrast, is the reverse reaction of mineralization. During immobilization, microorganisms convert inorganic forms to organic phosphate, which are then incorporated into their living cells. Mineralization and immobilization of phosphorus occur simultaneously in the soil. Ultimately, the C:P ratio determines whether there is net mineralization or net immobilization.
Factors affecting mineralization and immobilizationThe factors that affect P mineralization and immobilization are the same that affect nitrogen mineralization and immobilization:
Management of phosphorus—P-fixationP-fixation is a term that is used to describe both P-sorption and P precipitation. Since both P-sorption and P precipitation reduce phosphorus availability, a soil with a great P-fixation capacity has less available phosphorus after fertilization than a soil with a low P-fixation capacity. In other words, when the same amount of fertilizer is applied to a volcanic soil and a moderately weathered grassland soil, the volcanic soil has less P available due to its greater P-fixation capacity. How do we determine how much phosphorus to add?The answer is that we must account for the P-fixation capacity of the specific soil. For some Hawaii soils, researchers have determined the P-fixation capacity as various levels of phosphorus is added to the soil. This information allows us to predict how much phosphorus must be added to the soil to achieve a target phosphorus level. To view the P-sorption curves for selected soils, click on the link below: Phosphorus leaching and runoffIn Maui County and other tropical regions, highly weathered soils often provide little available phosphorus for plant growth. To further compound this issue, agricultural systems can experience phosphorus losses as the result of erosion by wind and runoff water. Erosion by wind can carry particles that contain sorbed-P to water systems, where phosphorus may later desorb. Sediments containing phosphorus can also contaminate ground and/or surface waters. Additionally, phosphorus availability is reduced by the removal of plant material (which can serve as a source of organic phosphorus) during harvest. Although phosphorus leaching is normally limited in most Hawaii soils due to their high P-fixing characteristics, phosphorus leaching can occur if the soil reaches maximum phosphorus holding capacity, especially when P fertilizers are overapplied. Sandy soils are most susceptible to phosphorus leaching. The consequence of phosphorus leaching is the contamination of ground water reserves. For further reading on the possible effects of excess phosphorus, click on the link below: |
|